Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane
- PMID: 27542209
- PMCID: PMC5312279
- DOI: 10.18632/oncotarget.11315
Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane
Abstract
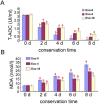
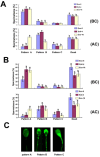
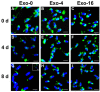
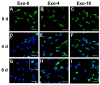
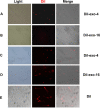
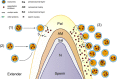
Seminal plasma ingredients are important for maintenance of sperm viability. This study focuses on the effect of boar seminal plasma exosomes on sperm function during long-term liquid storage. Boar seminal plasma exosomes had typical nano-structure morphology as measured by scanning electron microscopy (SEM) and molecular markers such as AWN, CD9 and CD63 by western blot analysis. The effect on sperm parameters of adding different ratio of boar seminal plasma exosomes to boar sperm preparations was analyzed. Compared to the diluent without exosomes, the diluent with four times or sixteen times exosomes compared to original semen had higher sperm motility, prolonged effective survival time, improved sperm plasma membrane integrity (p < 0.05), increased total antioxidant capacity (T-AOC) activity and decreased malondialdehyde (MDA) content. The diluent containing four times concentration of exosomes compared to original semen was determined to inhibit premature capacitation, but not to influence capacitation induced in vitro. Inhibition of premature capacitation is likely related to the concentration of exosomes which had been demonstrated to transfer proteins including AWN and PSP-1 into sperm. In addition, using fluorescence microscopy and scanning electron microscopy analysis, it was demonstrated that exosomes in diluent were directly binding to the membrane of sperm head which could improve sperm plasma membrane integrity.
Keywords: Pathology Section; boar sperm quality; capacitation; liquid storage; seminal plasma exosomes.
Conflict of interest statement
All authors read and approved the final manuscript. The authors declare no competing financial interests.
Figures


References
-
- Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell. 2015;163:1225–1236. - PubMed
-
- Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. The Prostate. 2009;69:159–167. - PubMed
-
- Suzuki K, Asano A, Eriksson B, Niwa K, Nagai T, Rodriguez-Martinez H. Capacitation status and in vitro fertility of boar spermatozoa: effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. International journal of andrology. 2002;25:84–93. - PubMed
-
- Abney TO, Williams WL. Inhibition of sperm capacitation by intrauterine deposition of seminal plasma decapacitation factor. Biology of reproduction. 1970;2:14–17. - PubMed
-
- Mendoza N, Casao A, Perez-Pe R, Cebrian-Perez JA, Muino-Blanco T. New insights into the mechanisms of ram sperm protection by seminal plasma proteins. Biology of reproduction. 2013;88:149. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

