A steroid like phytochemical Antcin M is an anti-aging reagent that eliminates hyperglycemia-accelerated premature senescence in dermal fibroblasts by direct activation of Nrf2 and SIRT-1
- PMID: 27542238
- PMCID: PMC5325331
- DOI: 10.18632/oncotarget.11229
A steroid like phytochemical Antcin M is an anti-aging reagent that eliminates hyperglycemia-accelerated premature senescence in dermal fibroblasts by direct activation of Nrf2 and SIRT-1
Abstract
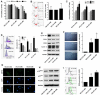
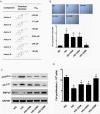
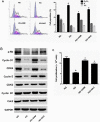
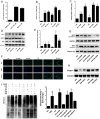
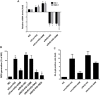
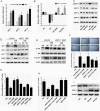
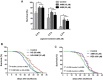
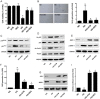
The present study revealed the anti-aging properties of antcin M (ANM) and elucidated the molecular mechanism underlying the effects. We found that exposure of human normal dermal fibroblasts (HNDFs) to high-glucose (HG, 30 mM) for 3 days, accelerated G0/G1 phase arrest and senescence. Indeed, co-treatment with ANM (10 µM) significantly attenuated HG-induced growth arrest and promoted cell proliferation. Further molecular analysis revealed that ANM blocked the HG-induced reduction in G1-S transition regulatory proteins such as cyclin D, cyclin E, CDK4, CDK6, CDK2 and protein retinoblastoma (pRb). In addition, treatment with ANM eliminated HG-induced reactive oxygen species (ROS) through the induction of anti-oxidant genes, HO-1 and NQO-1 via transcriptional activation of Nrf2. Moreover, treatment with ANM abolished HG-induced SIPS as evidenced by reduced senescence-associated β-galactosidase (SA-β-gal) activity. This effect was further confirmed by reduction in senescence-associated marker proteins including, p21CIP1, p16INK4A, and p53/FoxO1 acetylation. Also, the HG-induced decline in aging-related marker protein SMP30 was rescued by ANM. Furthermore, treatment with ANM increased SIRT-1 expression, and prevented SIRT-1 depletion. This protection was consistent with inhibition of SIRT-1 phosphorylation at Ser47 followed by blocking its upstream kinases, p38 MAPK and JNK/SAPK. Further analysis revealed that ANM partially protected HG-induced senescence in SIRT-1 silenced cells. A similar effect was also observed in Nrf2 silenced cells. However, a complete loss of protection was observed in both Nrf2 and SIRT-1 knockdown cells suggesting that both induction of Nrf2-mediated anti-oxidant defense and SIRT-1-mediated deacetylation activity contribute to the anti-aging properties of ANM in vitro. Result of in vivo studies shows that ANM-treated C. elegens exhibits an increased survival rate during HG-induced oxidative stress insult. Furthermore, ANM significantly extended the life span of C. elegans. Taken together, our results suggest the potential application of ANM in age-related diseases or as a preventive reagent against aging process.
Keywords: Antcin M; Gerotarget; SIRT-1; antrodia salmonea; hyperglycemia; stress-induced premature senescence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence.Biogerontology. 2018 Jul;19(3-4):237-249. doi: 10.1007/s10522-018-9753-9. Epub 2018 Mar 26. Biogerontology. 2018. PMID: 29582209
-
Phalaenopsis orchid flower extract attenuates high glucose-induced senescence via Nrf2/HO-1 activation and promotes wound healing in human dermal fibroblasts.Cell Mol Biol (Noisy-le-grand). 2025 Jul 6;71(6):110-118. doi: 10.14715/cmb/2025.71.6.15. Cell Mol Biol (Noisy-le-grand). 2025. PMID: 40618327
-
Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells.Oncotarget. 2017 Aug 4;8(57):96568-96587. doi: 10.18632/oncotarget.19951. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228553 Free PMC article.
-
Modulation of Sirtuins to address aging related disorders through the use of selected phytochemicals.Phytomedicine. 2025 Jul 25;143:156648. doi: 10.1016/j.phymed.2025.156648. Epub 2025 May 12. Phytomedicine. 2025. PMID: 40359853 Review.
-
The role of cellular senescence in Werner syndrome: toward therapeutic intervention in human premature aging.Ann N Y Acad Sci. 2007 Apr;1100:455-69. doi: 10.1196/annals.1395.051. Ann N Y Acad Sci. 2007. PMID: 17460211 Review.
Cited by
-
Astaxanthin protects oxidative stress mediated DNA damage and enhances longevity in Saccharomyces cerevisiae.Biogerontology. 2021 Feb;22(1):81-100. doi: 10.1007/s10522-020-09904-9. Epub 2020 Oct 27. Biogerontology. 2021. PMID: 33108581
-
Plant and fungal products that extend lifespan in Caenorhabditis elegans.Microb Cell. 2020 Jul 9;7(10):255-269. doi: 10.15698/mic2020.10.731. Microb Cell. 2020. PMID: 33015140 Free PMC article. Review.
-
Suppressing high mobility group box-1 release alleviates morphine tolerance via the adenosine 5'-monophosphate-activated protein kinase/heme oxygenase-1 pathway.Neural Regen Res. 2023 Sep;18(9):2067-2074. doi: 10.4103/1673-5374.366490. Neural Regen Res. 2023. PMID: 36926733 Free PMC article.
-
Mechanism and therapeutic potential of traditional Chinese medicine extracts in sepsis.Front Pharmacol. 2024 Jul 3;15:1365639. doi: 10.3389/fphar.2024.1365639. eCollection 2024. Front Pharmacol. 2024. PMID: 39021837 Free PMC article. Review.
-
Curcumin and o-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In Vitro.J Clin Med. 2019 Mar 29;8(4):433. doi: 10.3390/jcm8040433. J Clin Med. 2019. PMID: 30934902 Free PMC article.
References
-
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–45. - PubMed
-
- Levy L, Zeichner JA. Dermatologic manifestation of diabetes. J Diabetes. 2012;4:68–76. - PubMed
-
- Paron NG, Lambert PW. Cutaneous manifestations of diabetes mellitus. Prim Care. 2000;27:371–83. - PubMed
-
- Goldstein S, Niewiarowski S, Singal DP. Pathological implications of cell aging in vitro. Fed Proc. 1975;34:56–63. - PubMed
-
- Sibbitt WL, Jr, Mills RG, Bigler CF, Eaton RP, Griffey RH, Vander Jagt DL. Glucose inhibition of human fibroblast proliferation and response to growth factors is prevented by inhibitors of aldose reductase. Mech Ageing Develop. 1989;47:265–79. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

