Mechanistic Basis of Glutaminase Activation: A KEY ENZYME THAT PROMOTES GLUTAMINE METABOLISM IN CANCER CELLS
- PMID: 27542409
- PMCID: PMC5076503
- DOI: 10.1074/jbc.M116.720268
Mechanistic Basis of Glutaminase Activation: A KEY ENZYME THAT PROMOTES GLUTAMINE METABOLISM IN CANCER CELLS
Abstract
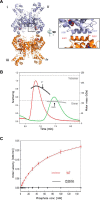
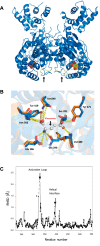
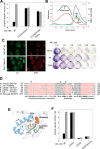
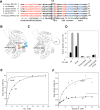
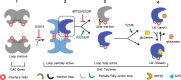
Glutamine-derived carbon becomes available for anabolic biosynthesis in cancer cells via the hydrolysis of glutamine to glutamate, as catalyzed by GAC, a splice variant of kidney-type glutaminase (GLS). Thus, there is significant interest in understanding the regulation of GAC activity, with the suggestion being that higher order oligomerization is required for its activation. We used x-ray crystallography, together with site-directed mutagenesis, to determine the minimal enzymatic unit capable of robust catalytic activity. Mutagenesis of the helical interface between the two pairs of dimers comprising a GAC tetramer yielded a non-active, GAC dimer whose x-ray structure displays a stationary loop ("activation loop") essential for coupling the binding of allosteric activators like inorganic phosphate to catalytic activity. Further mutagenesis that removed constraints on the activation loop yielded a constitutively active dimer, providing clues regarding how the activation loop communicates with the active site, as well as with a peptide segment that serves as a "lid" to close off the active site following substrate binding. Our studies show that the formation of large GAC oligomers is not a pre-requisite for full enzymatic activity. They also offer a mechanism by which the binding of activators like inorganic phosphate enables the activation loop to communicate with the active site to ensure maximal rates of catalysis, and promotes the opening of the lid to achieve optimal product release. Moreover, these findings provide new insights into how other regulatory events might induce GAC activation within cancer cells.
Keywords: enzyme mechanism; glutaminase; metabolism; mutagenesis; protein structure.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

