B cells in chronic obstructive pulmonary disease: moving to center stage
- PMID: 27542809
- PMCID: PMC5142126
- DOI: 10.1152/ajplung.00304.2016
B cells in chronic obstructive pulmonary disease: moving to center stage
Abstract
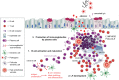
Chronic inflammatory responses in the lungs contribute to the development and progression of chronic obstructive pulmonary disease (COPD). Although research studies focused initially on the contributions of the innate immune system to the pathogenesis of COPD, more recent studies have implicated adaptive immune responses in COPD. In particular, studies have demonstrated increases in B cell counts and increases in the number and size of B cell-rich lymphoid follicles in COPD lungs that correlate directly with COPD severity. There are also increases in lung levels of mediators that promote B cell maturation, activation, and survival in COPD patients. B cell products such as autoantibodies directed against lung cells, components of cells, and extracellular matrix proteins are also present in COPD lungs. These autoantibodies may contribute to lung inflammation and injury in COPD patients, in part, by forming immune complexes that activate complement components. Studies of B cell-deficient mice and human COPD patients have linked B cells most strongly to the emphysema phenotype. However, B cells have protective activities during acute exacerbations of COPD by promoting adaptive immune responses that contribute to host defense against pathogens. This review outlines the evidence that links B cells and B cell-rich lymphoid follicles to the pathogenesis of COPD and the mechanisms involved. It also reviews the potential and limitations of B cells as therapeutic targets to slow the progression of human COPD.
Keywords: B cells; COPD; cigarette smoke; immunity; lymphoid follicles.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6: 205–217, 2006. - PubMed
-
- Baraldo S, Turato G, Lunardi F, Bazzan E, Schiavon M, Ferrarotti I, Molena B, Cazzuffi R, Damin M, Balestro E, Luisetti M, Rea F, Calabrese F, Cosio MG, Saetta M. Immune activation in alpha1-antitrypsin-deficiency emphysema. Beyond the protease-antiprotease paradigm. Am J Respir Crit Care Med 191: 402–409, 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

