THE POTENTIATION OF THE RADIOPROTECTIVE EFFICACY OF TWO MEDICAL COUNTERMEASURES, GAMMA-TOCOTRIENOL AND AMIFOSTINE, BY A COMBINATION PROPHYLACTIC MODALITY
- PMID: 27542813
- PMCID: PMC5444681
- DOI: 10.1093/rpd/ncw223
THE POTENTIATION OF THE RADIOPROTECTIVE EFFICACY OF TWO MEDICAL COUNTERMEASURES, GAMMA-TOCOTRIENOL AND AMIFOSTINE, BY A COMBINATION PROPHYLACTIC MODALITY
Abstract
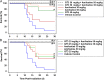
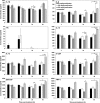
This study was designed to evaluate the possible potentiation of survival protection afforded by relatively low-dose amifostine prophylaxis against total body irradiation in combination with a protective, less toxic agent, gamma-tocotrienol (GT3). Mice were administered amifostine and/or GT3, then exposed to 9.2 Gy 60Co γ-irradiation and monitored for survival for 30 days. To investigate cytokine stimulation, mice were administered amifostine or GT3; serum samples were collected and analyzed for cytokines. Survival studies show single treatments of GT3 or amifostine significantly improved survival, compared to the vehicle, and combination treatments resulted in significantly higher survival compared to single treatments. In vivo studies with GT3 confirmed prior work indicating GT3 induces granulocyte colony-stimulating factor (G-CSF). This approach, the prophylactic combination of amifostine and GT3, which act through different mechanisms, shows promise and should be investigated further as a potential countermeasure for acute radiation syndrome.
© The Author 2016. Published by Oxford University Press.
Figures

Similar articles
-
Radioprotective Efficacy of Gamma-Tocotrienol in Nonhuman Primates.Radiat Res. 2016 Mar;185(3):285-98. doi: 10.1667/RR14127.1. Epub 2016 Mar 1. Radiat Res. 2016. PMID: 26930378
-
Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure.Cytokine. 2013 May;62(2):278-85. doi: 10.1016/j.cyto.2013.03.009. Epub 2013 Apr 3. Cytokine. 2013. PMID: 23561424
-
γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status.Int J Mol Sci. 2016 May 3;17(5):663. doi: 10.3390/ijms17050663. Int J Mol Sci. 2016. PMID: 27153057 Free PMC article. Review.
-
Development of Orally Administered γ-Tocotrienol (GT3) Nanoemulsion for Radioprotection.Int J Mol Sci. 2016 Dec 24;18(1):28. doi: 10.3390/ijms18010028. Int J Mol Sci. 2016. PMID: 28029115 Free PMC article.
-
Combining Pharmacological Countermeasures to Attenuate the Acute Radiation Syndrome-A Concise Review.Molecules. 2017 May 19;22(5):834. doi: 10.3390/molecules22050834. Molecules. 2017. PMID: 28534834 Free PMC article. Review.
Cited by
-
Repurposing Pharmaceuticals Previously Approved by Regulatory Agencies to Medically Counter Injuries Arising Either Early or Late Following Radiation Exposure.Front Pharmacol. 2021 May 10;12:624844. doi: 10.3389/fphar.2021.624844. eCollection 2021. Front Pharmacol. 2021. PMID: 34040517 Free PMC article. Review.
-
Histopathological studies of nonhuman primates exposed to supralethal doses of total- or partial-body radiation: influence of a medical countermeasure, gamma-tocotrienol.Sci Rep. 2024 Mar 8;14(1):5757. doi: 10.1038/s41598-024-56135-w. Sci Rep. 2024. PMID: 38459144 Free PMC article.
-
Pathology of acute sub-lethal or near-lethal irradiation of nonhuman primates prophylaxed with the nutraceutical, gamma tocotrienol.Sci Rep. 2024 Jun 10;14(1):13315. doi: 10.1038/s41598-024-64102-8. Sci Rep. 2024. PMID: 38858439 Free PMC article.
-
Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems.Expert Opin Pharmacother. 2020 Feb;21(3):317-337. doi: 10.1080/14656566.2019.1702968. Epub 2020 Jan 11. Expert Opin Pharmacother. 2020. PMID: 31928256 Free PMC article. Review.
-
Radioprotection and Radiomitigation: From the Bench to Clinical Practice.Biomedicines. 2020 Oct 30;8(11):461. doi: 10.3390/biomedicines8110461. Biomedicines. 2020. PMID: 33142986 Free PMC article. Review.
References
-
- U.S. Food and Drug Administration. About the pandemic and all-hazards preparedness reauthorization act of 2013 (PAHPRA), emergency preparedness and response: http://www.fda.gov/emergencypreparedness/medicalcountermeasures/ucm34619... (February 15, 2014).
-
- Hall E. J. and Giaccia A. J.. Radiobiology for the Radiobiologist (Philadelphia, PA, USA: Lippincott Williams and Wilkins; ) (2012) 978-0-60831-193-4.
-
- DiCarlo A. L., Maher C., Hick J. L., Hanfling D., Dainiak N., Chao N., Bader J. L., Coleman C. N. and Weinstock D. M.. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster. Med. Public. Health. Prep. 5(Suppl. 1), S32–44 (2011). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

