Peptides from American alligator plasma are antimicrobial against multi-drug resistant bacterial pathogens including Acinetobacter baumannii
- PMID: 27542832
- PMCID: PMC4992317
- DOI: 10.1186/s12866-016-0799-z
Peptides from American alligator plasma are antimicrobial against multi-drug resistant bacterial pathogens including Acinetobacter baumannii
Abstract
Background: Our group has developed a new process for isolating and identifying novel cationic antimicrobial peptides from small amounts of biological samples. Previously, we identified several active antimicrobial peptides from 100 μl of plasma from Alligator mississippiensis. These peptides were found to have in vitro antimicrobial activity against Pseudomonas aeruginosa and Staphylococcus aureus. In this work, we further characterize three of the novel peptides discovered using this process: Apo5, Apo6, and A1P.
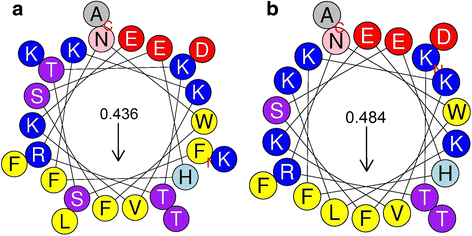
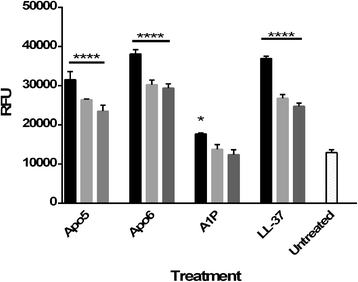
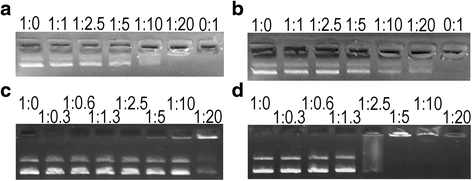
Results: We examined the activity of these peptides against multi-drug resistant strains and clinical isolates of common human pathogens. We investigated their structural characteristics using circular dichroism and tested for membrane disruption and DNA binding. These peptides were found to have strong in vitro activity against multi-drug resistant and clinically isolated strains of S. aureus, Escherichia coli, P. aeruginosa, and Acinetobacter baumannii. Apo5 and Apo6, peptides derived from alligator apolipoprotein C-1, depolarized the bacterial membrane. A1P, a peptide from the serpin proteinase inhibitor, did not permeabilize membranes. Performing circular dichroism analysis, Apo5 and Apo6 were found to be predominantly helical in SDS and TFE buffer, while A1P has significantly different structures in phosphate buffer, SDS, and TFE. None of these peptides were found to be hemolytic to sheep red blood cells or significantly cytotoxic up to 100 μg/ml after 24 h exposure.
Conclusions: Overall, we suggest that Apo5 and Apo6 have a different mode of action than A1P, and that all three peptides make promising candidates for the treatment of drug-resistant bacteria, such as A. baumannii.
Keywords: Acinetobacter baumannii; Alligator mississippiensis; Antimicrobial peptides; Escherichia coli; Multi-drug resistant bacteria; Staphylococcus aureus.
Figures



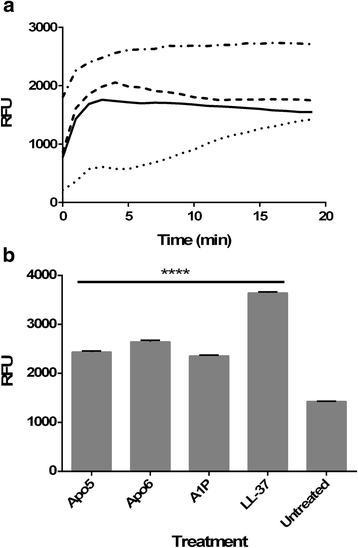
 ), 0.5 μg/ml (
), 0.5 μg/ml ( ), as well as no treatment (
), as well as no treatment ( )
)

 ), A1P (
), A1P ( ), LL-37 (
), LL-37 ( )
)Similar articles
-
Antimicrobial activities and membrane-active mechanism of CPF-C1 against multidrug-resistant bacteria, a novel antimicrobial peptide derived from skin secretions of the tetraploid frog Xenopus clivii.J Pept Sci. 2014 Nov;20(11):876-84. doi: 10.1002/psc.2679. Epub 2014 Aug 6. J Pept Sci. 2014. PMID: 25098547
-
Antimicrobial activities and action mechanism studies of transportan 10 and its analogues against multidrug-resistant bacteria.J Pept Sci. 2015 Jul;21(7):599-607. doi: 10.1002/psc.2781. Epub 2015 Apr 16. J Pept Sci. 2015. PMID: 25891396
-
Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae.Dev Comp Immunol. 2017 May;70:135-144. doi: 10.1016/j.dci.2017.01.011. Epub 2017 Jan 13. Dev Comp Immunol. 2017. PMID: 28089718
-
Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections.Microb Pathog. 2020 Sep;146:104238. doi: 10.1016/j.micpath.2020.104238. Epub 2020 May 5. Microb Pathog. 2020. PMID: 32387392 Review.
-
Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy.Expert Rev Anti Infect Ther. 2010 Jan;8(1):71-93. doi: 10.1586/eri.09.108. Expert Rev Anti Infect Ther. 2010. PMID: 20014903 Review.
Cited by
-
The Komodo dragon (Varanus komodoensis) genome and identification of innate immunity genes and clusters.BMC Genomics. 2019 Aug 30;20(1):684. doi: 10.1186/s12864-019-6029-y. BMC Genomics. 2019. PMID: 31470795 Free PMC article.
-
Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options.Front Cell Infect Microbiol. 2017 Mar 13;7:55. doi: 10.3389/fcimb.2017.00055. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28348979 Free PMC article. Review.
-
Post-Translational Protein Deimination Signatures in Plasma and Plasma EVs of Reindeer (Rangifer tarandus).Biology (Basel). 2021 Mar 13;10(3):222. doi: 10.3390/biology10030222. Biology (Basel). 2021. PMID: 33805829 Free PMC article.
-
Exploring the Gastrointestinal Microbiome of Eurasian Griffon Vultures (Gyps fulvus) Under Rehabilitation in Portugal and Their Potential Role as Reservoirs of Human and Animal Pathogens.Vet Sci. 2024 Dec 4;11(12):622. doi: 10.3390/vetsci11120622. Vet Sci. 2024. PMID: 39728962 Free PMC article.
-
Anti-Pseudomonas aeruginosa activity of natural antimicrobial peptides when used alone or in combination with antibiotics.Front Microbiol. 2023 Sep 5;14:1239540. doi: 10.3389/fmicb.2023.1239540. eCollection 2023. Front Microbiol. 2023. PMID: 37731929 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

