Prolactin-induced PAK1 tyrosyl phosphorylation promotes FAK dephosphorylation, breast cancer cell motility, invasion and metastasis
- PMID: 27542844
- PMCID: PMC4992334
- DOI: 10.1186/s12860-016-0109-5
Prolactin-induced PAK1 tyrosyl phosphorylation promotes FAK dephosphorylation, breast cancer cell motility, invasion and metastasis
Abstract
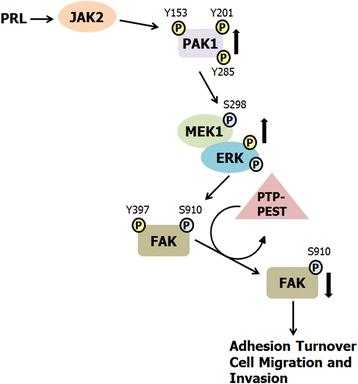
Background: The serine/threonine kinase PAK1 is an important regulator of cell motility. Both PAK1 and the hormone/cytokine prolactin (PRL) have been implicated in breast cancer cell motility, however, the exact mechanisms guiding PRL/PAK1 signaling in breast cancer cells have not been fully elucidated. Our lab has previously demonstrated that PRL-activated tyrosine kinase JAK2 phosphorylates PAK1 on tyrosines 153, 201, and 285, and that tyrosyl phosphorylated PAK1 (pTyr-PAK1) augments migration and invasion of breast cancer cells.
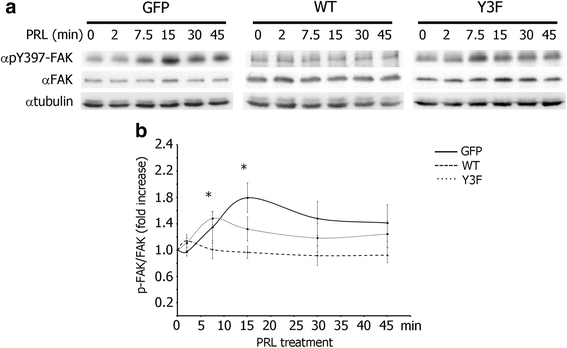
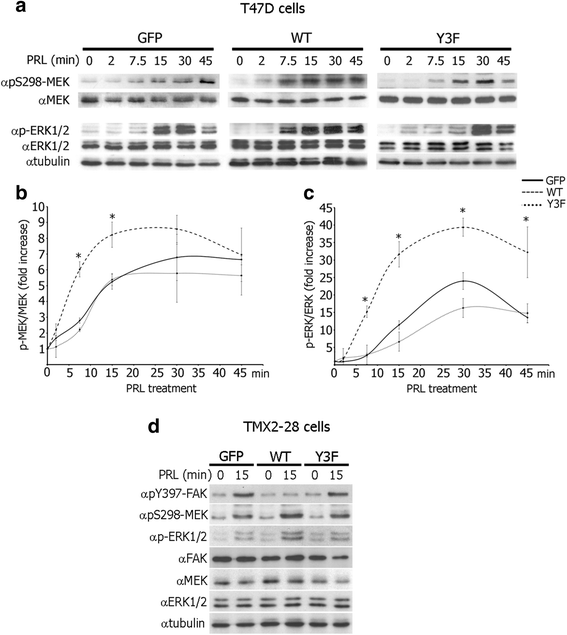
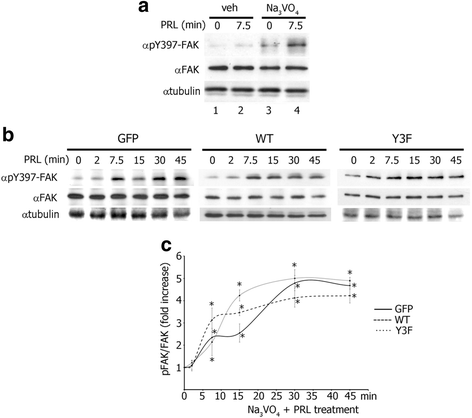
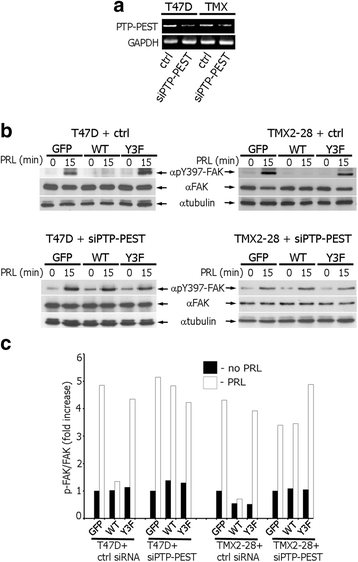
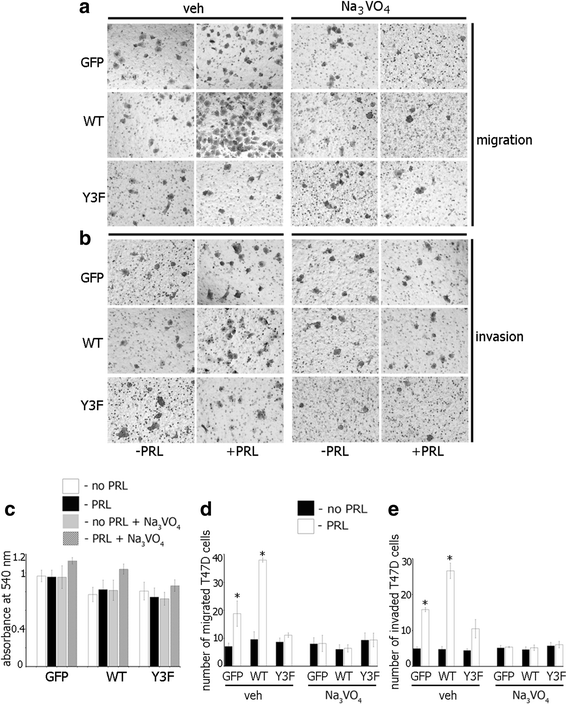
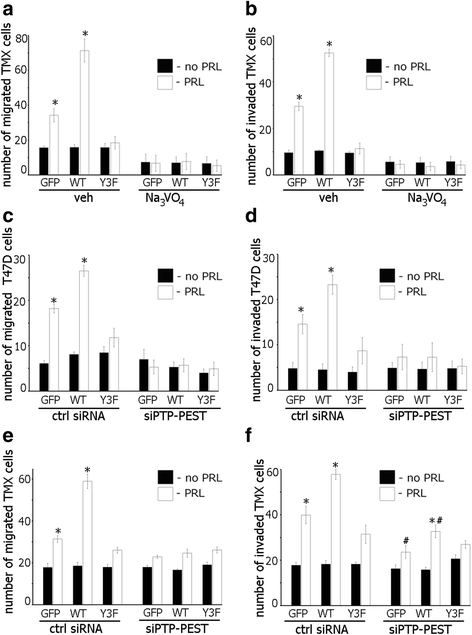
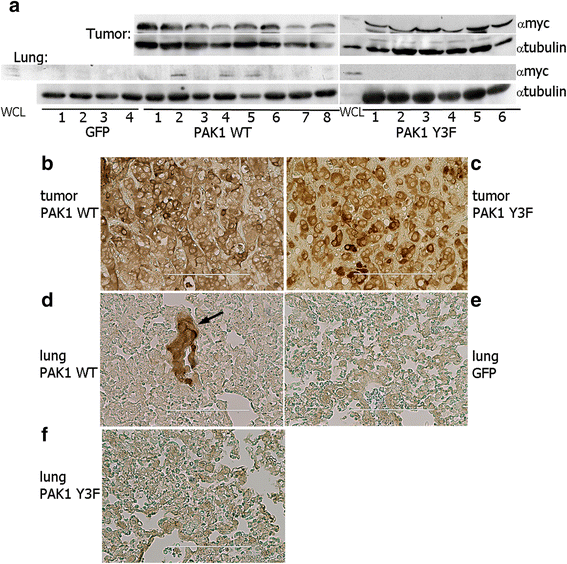
Results: Here we further investigate the mechanisms by which pTyr-PAK1 enhances breast cancer cell motility in response to PRL. We demonstrate a distinct reduction in PRL-induced FAK auto-phosphorylation in T47D and TMX2-28 breast cancer cells overexpressing wild-type PAK1 (PAK1 WT) when compared to cells overexpressing either GFP or phospho-tyrosine-deficient mutant PAK1 (PAK1 Y3F). Furthermore, pTyr-PAK1 phosphorylates MEK1 on Ser298 resulting in subsequent ERK1/2 activation. PRL-induced FAK auto-phosphorylation is rescued in PAK1 WT cells by inhibiting tyrosine phosphatases and tyrosine phosphatase inhibition abrogates cell motility and invasion in response to PRL. siRNA-mediated knockdown of the tyrosine phosphatase PTP-PEST rescues FAK auto-phosphorylation in PAK1 WT cells and reduces both cell motility and invasion. Finally, we provide evidence that PRL-induced pTyr-PAK1 stimulates tumor cell metastasis in vivo.
Conclusion: These data provide insight into the mechanisms guiding PRL-mediated breast cancer cell motility and invasion and highlight a significant role for pTyr-PAK1 in breast cancer metastasis.
Keywords: Breast cancer cells; FAK; PAK1; Prolactin; Tyrosyl phosphorylation.
Figures
Similar articles
-
Tyrosyl phosphorylated PAK1 regulates breast cancer cell motility in response to prolactin through filamin A.Mol Endocrinol. 2013 Mar;27(3):455-65. doi: 10.1210/me.2012-1291. Epub 2013 Jan 22. Mol Endocrinol. 2013. PMID: 23340249 Free PMC article.
-
Tyrosyl phosphorylated serine-threonine kinase PAK1 is a novel regulator of prolactin-dependent breast cancer cell motility and invasion.Adv Exp Med Biol. 2015;846:97-137. doi: 10.1007/978-3-319-12114-7_5. Adv Exp Med Biol. 2015. PMID: 25472536 Free PMC article. Review.
-
PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV.Mol Endocrinol. 2013 Jul;27(7):1048-64. doi: 10.1210/me.2012-1322. Epub 2013 Jun 6. Mol Endocrinol. 2013. PMID: 23744893 Free PMC article.
-
Phosphorylation of tyrosine 285 of PAK1 facilitates βPIX/GIT1 binding and adhesion turnover.FASEB J. 2015 Mar;29(3):943-59. doi: 10.1096/fj.14-259366. Epub 2014 Dec 2. FASEB J. 2015. PMID: 25466889 Free PMC article.
-
Involvement of the PRL-PAK1 Pathway in Cancer Cell Migration.Cancer Diagn Progn. 2023 Jan 3;3(1):17-25. doi: 10.21873/cdp.10174. eCollection 2023 Jan-Feb. Cancer Diagn Progn. 2023. PMID: 36632591 Free PMC article. Review.
Cited by
-
Prolactin and endocrine therapy resistance in breast cancer: The next potential hope for breast cancer treatment.J Cell Mol Med. 2021 Nov;25(22):10327-10348. doi: 10.1111/jcmm.16946. Epub 2021 Oct 15. J Cell Mol Med. 2021. PMID: 34651424 Free PMC article. Review.
-
Prolactin-activated PAK1 potentiates estrogen response to breast cancer cell epithelial-mesenchymal transition, migration and invasion.MicroPubl Biol. 2024 Jun 11;2024:10.17912/micropub.biology.001195. doi: 10.17912/micropub.biology.001195. eCollection 2024. MicroPubl Biol. 2024. PMID: 38933712 Free PMC article.
-
Silencing TRIM37 inhibits the proliferation and migration of non-small cell lung cancer cells.RSC Adv. 2018 Oct 31;8(64):36852-36857. doi: 10.1039/c8ra06391e. eCollection 2018 Oct 26. RSC Adv. 2018. PMID: 35558931 Free PMC article.
-
The Relevant Participation of Prolactin in the Genesis and Progression of Gynecological Cancers.Front Endocrinol (Lausanne). 2021 Oct 21;12:747810. doi: 10.3389/fendo.2021.747810. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34745013 Free PMC article. Review.
-
Prolactin-induced tyrosyl phosphorylation of PAK1 facilitates epithelial-mesenchymal transition.MicroPubl Biol. 2024 Apr 9;2024:10.17912/micropub.biology.001136. doi: 10.17912/micropub.biology.001136. eCollection 2024. MicroPubl Biol. 2024. PMID: 38660565 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

