Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study
- PMID: 27542891
- PMCID: PMC5155651
- DOI: 10.1111/irv.12421
Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study
Abstract
Aim: Aspirin (acetylsalicylic acid) has been used for more than 115 years in medicine. Research exists to show that aspirin has antiviral effects in vitro, for example, by blocking influenza virus propagation via NF-κB inhibition when used at high concentrations and short-term incubation steps. The aim of this study was to confirm the antiviral activity of aspirin against influenza virus and further elucidate the activity of aspirin against other respiratory viruses.
Methods: Tests to detect antiviral activity were performed using plaque-reduction assays. Aspirin was administered to the virus-infected cell cultures one hour after infection. Prior to these assays, the non-cytotoxic concentrations of aspirin on cells used for propagation of the respective viruses were determined.
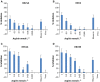
Results: Aspirin was found to be highly effective against influenza A H1N1 virus. The antiviral activity against further respiratory RNA viruses was less distinct. Respiratory syncytial virus was minimally inhibited. However, the activity of aspirin against rhinoviruses was more pronounced. Aspirin demonstrated antiviral activity against all human rhinoviruses (HRV), but the effect on members of the "major group" viruses, namely HRV14 and HRV39, was greater than on those of the "minor group," HRV1A and HRV2.
Conclusions: These data demonstrate a specific antiviral activity of aspirin against influenza A virus and HRV. The mode of action against rhinoviruses is still unknown and requires further investigation, as does the possibility of aspirin being effective in vivo to treat the common cold.
Keywords: acetylsalicylic acid; antiviral activity; aspirin; influenza; plaque-reduction assay; rhinoviruses.
© 2016 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures


References
-
- Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with Acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, single‐dose, 6 hour dose‐ranging study. Clin Ther. 2005;27:993–1003. - PubMed
-
- Brayfield A. Aspirin. The Complete Drug Reference. 38th edn. Martindale: Pharmaceutical Press; 2014.
-
- Morris T, Stables M, Hobbs A, et al. Effects of low‐dose Aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

