Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle
- PMID: 27542947
- PMCID: PMC5099996
- DOI: 10.1093/molehr/gaw041
Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle
Abstract
Study question: Do interactions between human fallopian tube epithelium and murine follicles occur during an artificial reproductive cycle in a co-culture system in vitro?
Summary answer: In a co-culture system, human fallopian tissues responded to the menstrual cycle mimetic by changes in morphology and levels of secreted factors, and increasing murine corpus luteum progesterone secretion.
What is known already: The entire fallopian tube epithelium, including ciliated and secretory cells, can be regulated in the reproductive cycle. Currently, there are no in vitro culture models that can monitor fallopian tissues in real time in response to factors produced by the ovary. In addition, there are no reports on the impact of fallopian tissue on ovarian function during the menstrual cycle.
Study design, samples/materials, methods: Human fallopian tissue (n = 24) was obtained by routine hysterectomies from women (aged 26-50 years, mean age = 43.6) who had not undergone exogenous hormonal treatment for at least 3 months prior to surgery. CD1 female mice were used for ovarian follicle isolation. The human fallopian epithelium layers were either co-cultured with five murine multilayer secondary follicles (150-180 μm follicles, encapsulated in one alginate gel bead) for 15 days or received stepwise steroid hormone additions for 13 days. The fallopian tissue morphology and cilia beating rate, as measured by an Andor Spinning Disk Confocal, were investigated. Oviduct-specific glycoprotein 1 (OVGP1), human insulin-like growth factor 1 (hIGF1), vascular endothelial growth factor A (VEGF-A) and interleukin 8 (IL8) as biological functional markers were measured either by ELISA or western blot to indicate dynamic changes in the fallopian epithelium during the reproductive cycle generated by mouse follicles or by stepwise steroid hormone induction. Three or four patients in each experiment were recruited for replicates. Data were presented as mean ± SD and further analyzed using one-way ANOVA followed by Tukey's multiple comparisons test.
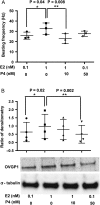
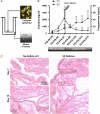
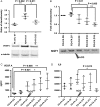
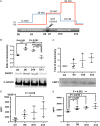
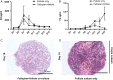
Main results and the role of chance: The cultured fallopian tube epithelium responded to exogenous steroid hormone stimulation, as demonstrated by enhanced cilia beating rate (~25% increase, P = 0.04) and an increase in OVGP1 secretion (P = 0.02) in response to 1 nM estradiol (E2) treatment when compared with 0.1 nM E2. Conversely, 10 nM progesterone plus 1 nM E2 suppressed cilia beating rate by ~30% (P = 0.008), while OVGP1 secretion was suppressed by 0.1 nM E2 plus 50 nM progesterone (P = 0.002 versus 1 nM E2 alone). Human fallopian tube epithelium was co-cultured with murine secondary follicles to mimic the human menstrual cycle. OVGP1 and VEGF-A secretion from fallopian tissue was similar with stepwise hormone treatment and when cultured with murine follicles. However, the secretion patterns of hIGF1 and IL8 differed in the luteal phase when comparing steroid treatment with follicle co-culture. In co-culture, hIGF1 secretion was suppressed in the luteal versus follicular phase (P = 0.005) but stepwise hormone treatment had no effect on hIGF1. In co-culture, IL8 secretion was also suppressed on luteal phase day 15 (P = 0.013) versus follicular phase day 7, but IL8 secretion increased continuously under high E2/progesterone treatment (P = 0.003 for D13 versus D3). In the co-culture system, the corpus luteum continuously produced progesterone in the presence of fallopian tube tissue until Day 18 while, without fallopian tissue, progesterone started to drop from Day 13.
Limitations, reasons for caution: One limitation of this study is that murine follicles were used to mimic the human menstrual cycle. However, although secretion patterns of peptide hormones such as inhibins and activins differ in mice and humans, the co-culture system used here did reveal interactions between the tissues that govern reproductive function.
Wider implications of the findings: In vitro co-culture models of fallopian reproductive tissues with ovarian follicles can provide an important tool for understanding fertility and for uncovering the mechanisms responsible for reduced fertility. In addition, the role of oviductal secretions and how they influence ovarian function, such as the production of progesterone during the menstrual cycle, can be uncovered using this model.
Large-scale data: None.
Study funding and competing interests: This work was funded by grants from the NIH (UH3TR001207), the American Cancer Society (RSG-12-230-01-TBG) and NIH (R01EB014806). The authors declare no competing financial interest.
Keywords: co-culture; fallopian tube; follicle; in vitro culture; luteal deficiency; menstrual cycle.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Engineering the ovarian cycle using in vitro follicle culture.Hum Reprod. 2015 Jun;30(6):1386-95. doi: 10.1093/humrep/dev052. Epub 2015 Mar 16. Hum Reprod. 2015. PMID: 25784584 Free PMC article.
-
Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture.Hum Reprod. 2013 Aug;28(8):2187-200. doi: 10.1093/humrep/det093. Epub 2013 Apr 21. Hum Reprod. 2013. PMID: 23608357 Free PMC article.
-
Anti-Müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates.Hum Reprod. 2016 Jul;31(7):1522-30. doi: 10.1093/humrep/dew100. Epub 2016 May 9. Hum Reprod. 2016. PMID: 27165618 Free PMC article.
-
The neuroendocrine regulation of the human ovarian cycle.Chronobiol Int. 2001 Nov;18(6):893-919. Chronobiol Int. 2001. PMID: 11777079 Review.
-
Physiology of the menstrual cycle.Am J Clin Nutr. 1975 Apr;28(4):333-8. doi: 10.1093/ajcn/28.4.333. Am J Clin Nutr. 1975. PMID: 1091131 Review.
Cited by
-
Tissue chips - innovative tools for drug development and disease modeling.Lab Chip. 2017 Sep 12;17(18):3026-3036. doi: 10.1039/c7lc00462a. Lab Chip. 2017. PMID: 28795174 Free PMC article. Review.
-
Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis.Front Endocrinol (Lausanne). 2022 Jul 29;13:936765. doi: 10.3389/fendo.2022.936765. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35966050 Free PMC article.
-
A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle.Nat Commun. 2017 Mar 28;8:14584. doi: 10.1038/ncomms14584. Nat Commun. 2017. PMID: 28350383 Free PMC article.
-
Bioengineering models of female reproduction.Biodes Manuf. 2020 Sep;3(3):237-251. doi: 10.1007/s42242-020-00082-8. Epub 2020 Jun 16. Biodes Manuf. 2020. PMID: 32774987 Free PMC article.
-
High-Fat Diet and Female Fertility across Lifespan: A Comparative Lesson from Mammal Models.Nutrients. 2022 Oct 17;14(20):4341. doi: 10.3390/nu14204341. Nutrients. 2022. PMID: 36297035 Free PMC article. Review.
References
-
- Alvarez-Sanchez F, Segal SJ, Brache V, Adejuwon CA, Leon P, Faundes A.. Pituitary-ovarian function after tubal ligation. Fertil Steril 1981;36:606–609. - PubMed
-
- Balasubramaniam ES, Van Noorden S, El-Bahrawy M.. The expression of interleukin (IL)-6, IL-8, and their receptors in fallopian tubes with ectopic tubal gestation. Fertil Steril 2012;98:898–904. - PubMed
-
- Bauersachs S, Rehfeld S, Ulbrich SE, Mallok S, Prelle K, Wenigerkind H, Einspanier R, Blum H, Wolf E.. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J Mol Endocrinol 2004;32:449–466. - PubMed
-
- Briton-Jones C, Lok IH, Cheung CK, Chiu TTY, Cheung LP, Haines C.. Estradiol regulation of oviductin/oviduct-specific glycoprotein messenger ribonucleic acid expression in human oviduct mucosal cells in vitro. Fertil Steril 2004;81:749–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

