Inducible and naturally occurring regulatory T cells enhance lung allergic responses through divergent transcriptional pathways
- PMID: 27542981
- PMCID: PMC5313386
- DOI: 10.1016/j.jaci.2016.06.051
Inducible and naturally occurring regulatory T cells enhance lung allergic responses through divergent transcriptional pathways
Abstract
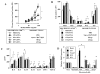
Background: Regulatory T cells attenuate development of asthma in wild-type (WT) mice, with both naturally occurring regulatory T (nTreg) cells and inducible regulatory T (iTreg) cells exhibiting suppressive activity. When transferred into CD8-deficient (CD8-/-) recipients, both cell types enhanced development of allergen-induced airway hyperresponsiveness.
Objective: We sought to determine whether the pathways leading to enhancement of lung allergic responses by transferred nTreg and iTreg cells differed.
Methods: nTreg cells (CD4+CD25+) were isolated from WT mice and iTreg cells were generated from WT CD4+CD25- T cells after activation in the presence of TGF-β and transferred into sensitized CD8-/- recipients before challenge. Development of airway hyperresponsiveness, cytokine levels, and airway inflammation were monitored.
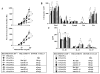
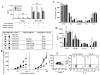
Results: Transfer of nTreg cells enhanced lung allergic responses, as did transfer of iTreg cells. Although anti-IL-13 reduced nTreg cell-mediated enhancement, it was ineffective in iTreg cell-mediated enhancement; conversely, anti-IL-17, but not anti-IL-13, attenuated the enhancement by iTreg cells. Recovered iTreg cells from the lungs of CD8-/- recipients were capable of IL-17 production and expressed high levels of signature genes of the TH17 pathway, RORγt and Il17, whereas reduced expression of the Treg cell key transcription factor forkhead box p3 (Foxp3) was observed. In vitro exogenous IL-6-induced IL-17 production in iTreg cells, and in vivo conversion of transferred iTreg cells was dependent on recipient IL-6.
Conclusions: iTreg cells, similar to nTreg cells, exhibit functional plasticity and can be converted from suppressor cells to pathogenic effector cells, enhancing lung allergic responses, but these effects were mediated through different pathways.
Keywords: IL-13; IL-17; Inducible and naturally occurring regulatory T cells; asthma; enhancement; suppression.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Dichotomous role of TGF-β controls inducible regulatory T-cell fate in allergic airway disease through Smad3 and TGF-β-activated kinase 1.J Allergy Clin Immunol. 2020 Mar;145(3):933-946.e4. doi: 10.1016/j.jaci.2019.09.032. Epub 2019 Oct 15. J Allergy Clin Immunol. 2020. PMID: 31626843 Free PMC article.
-
Comparison of induced versus natural regulatory T cells of the same TCR specificity for induction of tolerance to an environmental antigen.J Immunol. 2013 Aug 1;191(3):1136-43. doi: 10.4049/jimmunol.1201899. Epub 2013 Jul 1. J Immunol. 2013. PMID: 23817420
-
Programmed Death-1 antibody blocks therapeutic effects of T-regulatory cells in cockroach antigen-induced allergic asthma.Am J Respir Cell Mol Biol. 2010 Oct;43(4):432-42. doi: 10.1165/rcmb.2009-0258OC. Epub 2009 Nov 9. Am J Respir Cell Mol Biol. 2010. PMID: 19901343 Free PMC article.
-
TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea for asthma.Immunol Res. 2006;35(3):219-32. doi: 10.1385/IR:35:3:219. Immunol Res. 2006. PMID: 17172648 Review.
-
Expanding and converting regulatory T cells: a horizon for immunotherapy.Arch Immunol Ther Exp (Warsz). 2009 May-Jun;57(3):199-204. doi: 10.1007/s00005-009-0021-1. Epub 2009 May 29. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19479206 Review.
Cited by
-
Reduced CCR6+IL-17A+Treg Cells in Blood and CCR6-Dependent Accumulation of IL-17A+Treg Cells in Lungs of Patients With Allergic Asthma.Front Immunol. 2021 Aug 23;12:710750. doi: 10.3389/fimmu.2021.710750. eCollection 2021. Front Immunol. 2021. PMID: 34497608 Free PMC article.
-
The role of basophils as innate immune regulatory cells in allergy and immunotherapy.Hum Vaccin Immunother. 2018 Apr 3;14(4):815-831. doi: 10.1080/21645515.2017.1417711. Epub 2018 Jan 18. Hum Vaccin Immunother. 2018. PMID: 29257936 Free PMC article. Review.
-
Dihydroartemisinin Ameliorated Ovalbumin-Induced Asthma in Mice via Regulation of MiR-183C.Med Sci Monit. 2019 May 22;25:3804-3814. doi: 10.12659/MSM.915399. Med Sci Monit. 2019. PMID: 31115390 Free PMC article.
-
Forkhead box protein 3 demethylation is associated with tolerance induction in peanut-induced intestinal allergy.J Allergy Clin Immunol. 2018 Feb;141(2):659-670.e2. doi: 10.1016/j.jaci.2017.04.020. Epub 2017 May 4. J Allergy Clin Immunol. 2018. PMID: 28479331 Free PMC article.
-
Spectrum of T-lymphocyte activities regulating allergic lung inflammation.Immunol Rev. 2017 Jul;278(1):63-86. doi: 10.1111/imr.12561. Immunol Rev. 2017. PMID: 28658551 Free PMC article. Review.
References
-
- Bennet CL, Christie J, Ramsdell F, Brunkow ME, Fergurson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome is caused mutations of Foxp3. Nature Genet. 2001;27:20–1. - PubMed
-
- Clark LB, Appleby MW, Brunkow ME, Willkinson JE, Ziegler SF, Ramshell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. - PubMed
-
- Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. 2007;178:7667–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

