Ubiquitous miR159 repression of MYB33/65 in Arabidopsis rosettes is robust and is not perturbed by a wide range of stresses
- PMID: 27542984
- PMCID: PMC4992245
- DOI: 10.1186/s12870-016-0867-4
Ubiquitous miR159 repression of MYB33/65 in Arabidopsis rosettes is robust and is not perturbed by a wide range of stresses
Abstract
Background: The microR159 (miR159) - GAMYB pathway is conserved in higher plants, where GAMYB, expression promotes programmed cell death in seeds (aleurone) and anthers (tapetum). In cereals, restriction of GAMYB expression to seeds and anthers is mainly achieved transcriptionally, whereas in Arabidopsis this is achieved post-transcriptionally, as miR159 silences GAMYB (MYB33 and MYB65) in vegetative tissues, but not in seeds and anthers. However, we cannot rule out a role for miR159-MYB33/65 pathway in Arabidopsis vegetative tissues; a loss-of-function mir159 Arabidopsis mutant displays strong pleiotropic defects and numerous reports have documented changes in miR159 abundance during stress and hormone treatments. Hence, we have investigated the functional role of this pathway in vegetative tissues.
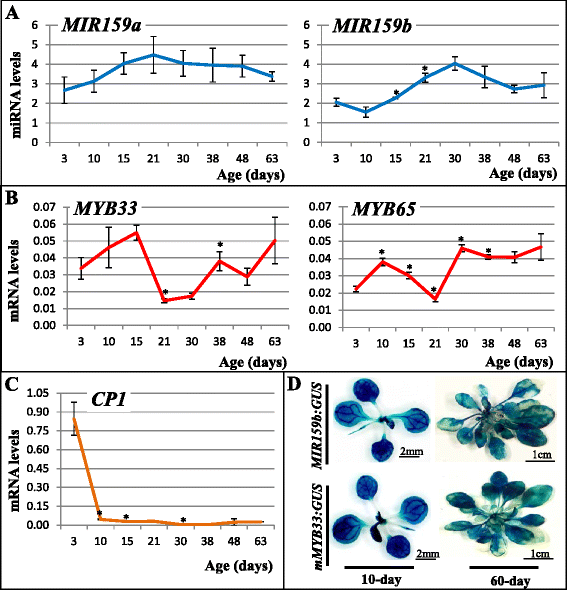
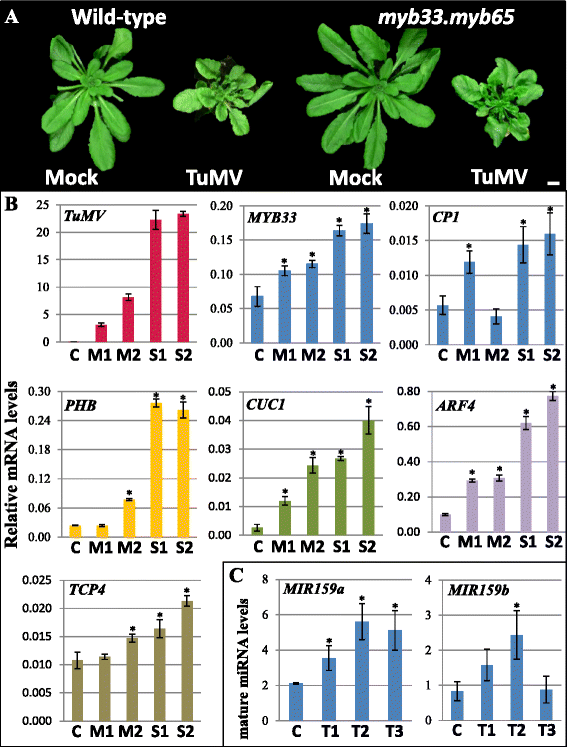
Results: It was found that the miR159-MYB33/65 pathway was ubiquitously present throughout rosette development. However, miR159 appears to continuously repress MYB33/MYB65 expression to levels that have no major impact on rosette development. Inducible inhibition of miR159 resulted in MYB33/65 de-repression and associated phenotypic defects, indicating that a potential role in vegetative development is only possible through MYB33 and MYB65 if miR159 levels decrease. However, miR159 silencing of MYB33/65 appeared extremely robust; no tested abiotic stress resulted in strong miR159 repression. Consistent with this, the stress responses of an Arabidopsis mutant lacking the miR159-MYB33/65 pathway were indistinguishable from wild-type. Moreover, expression of viral silencing suppressors, either via transgenesis or viral infection, was unable to prevent miR159 repression of MYB33/65, highlighting the robustness of miR159-mediated silencing.
Conclusions: Despite being ubiquitously present, molecular, genetic and physiological analysis failed to find a major functional role for the miR159-MYB33/65 pathway in Arabidopsis rosette development or stress response. Although it is likely that this pathway is important for a stress not tested here or in different plant species, our findings argue against the miR159-MYB33/65 pathway playing a major conserved role in general stress response. Finally, in light of the robustness of miR159-mediated repression of MYB33/65, it appears unlikely that low fold-level changes of miR159 abundance in response to stress would have any major physiological impact in Arabidopsis.
Keywords: Arabidopsis; GAMYB-like; Stress; Viral silencing suppressors; miR159.
Figures




Similar articles
-
The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis.Plant Physiol. 2010 Oct;154(2):757-71. doi: 10.1104/pp.110.160630. Epub 2010 Aug 10. Plant Physiol. 2010. PMID: 20699403 Free PMC article.
-
MicroRNA159 can act as a switch or tuning microRNA independently of its abundance in Arabidopsis.PLoS One. 2012;7(4):e34751. doi: 10.1371/journal.pone.0034751. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511963 Free PMC article.
-
Determinants beyond both complementarity and cleavage govern microR159 efficacy in Arabidopsis.PLoS Genet. 2014 Mar 13;10(3):e1004232. doi: 10.1371/journal.pgen.1004232. eCollection 2014 Mar. PLoS Genet. 2014. PMID: 24626050 Free PMC article.
-
Repression of miR156 by miR159 Regulates the Timing of the Juvenile-to-Adult Transition in Arabidopsis.Plant Cell. 2017 Jun;29(6):1293-1304. doi: 10.1105/tpc.16.00975. Epub 2017 May 23. Plant Cell. 2017. PMID: 28536099 Free PMC article.
-
Biology and Function of miR159 in Plants.Plants (Basel). 2019 Jul 30;8(8):255. doi: 10.3390/plants8080255. Plants (Basel). 2019. PMID: 31366066 Free PMC article. Review.
Cited by
-
Dynamics of small RNAs in a red-fruited wine grape cultivar infected with Grapevine red blotch virus.BMC Genomics. 2025 Apr 29;26(1):417. doi: 10.1186/s12864-025-11539-4. BMC Genomics. 2025. PMID: 40301705 Free PMC article.
-
MIR159 regulates multiple aspects of stamen and carpel development and requires dissection and delimitation of differential downstream regulatory network for manipulating fertility traits.Physiol Mol Biol Plants. 2023 Oct;29(10):1437-1456. doi: 10.1007/s12298-023-01377-7. Epub 2023 Nov 6. Physiol Mol Biol Plants. 2023. PMID: 38076769 Free PMC article.
-
LoBLH6 interacts with LoMYB65 to regulate anther development through feedback regulation of gibberellin synthesis in lily.Hortic Res. 2024 Dec 4;12(3):uhae339. doi: 10.1093/hr/uhae339. eCollection 2025 Mar. Hortic Res. 2024. PMID: 40061809 Free PMC article.
-
Transcriptome, miRNA, and degradome sequencing reveal the leaf stripe (Pyrenophora graminea) resistance genes in Tibetan hulless barley.BMC Plant Biol. 2025 Jan 17;25(1):71. doi: 10.1186/s12870-025-06055-2. BMC Plant Biol. 2025. PMID: 39825242 Free PMC article.
-
Integrated microRNA and transcriptome profiling reveals a miRNA-mediated regulatory network of embryo abortion under calcium deficiency in peanut (Arachis hypogaea L.).BMC Genomics. 2019 May 21;20(1):392. doi: 10.1186/s12864-019-5770-6. BMC Genomics. 2019. PMID: 31113378 Free PMC article.
References
-
- Tsuji H, Aya K, Ueguchi-Tanaka M, Shimada Y, Nakazono M, Watanabe R, Nishizawa NK, Gomi K, Shimada A, Kitano H, Ashikari M, Matsuoka M. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 2006;47:427–44. doi: 10.1111/j.1365-313X.2006.02795.x. - DOI - PubMed
-
- Palatnik JF, Wollmann H, Schommer C, Schwab R, Boisbouvier J, Rodriguez R, Warthmann N, Allen E, Dezulian T, Huson D, Carrington JC, Weigel D. Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Dev Cell. 2007;13:115–25. doi: 10.1016/j.devcel.2007.04.012. - DOI - PubMed
-
- Csukasi F, Donaire L, Casañal A, Martínez-Priego L, Botella MA, Medina-Escobar N, Llave C, Valpuesta V. Two strawberry miR159 family members display developmental-specific expression patterns in the fruit receptacle and cooperatively regulate Fa-GAMYB. New Phytol. 2012;195:47–57. doi: 10.1111/j.1469-8137.2012.04134.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

