Medullary thymic epithelial cells and CD8α+ dendritic cells coordinately regulate central tolerance but CD8α+ cells are dispensable for thymic regulatory T cell production
- PMID: 27543048
- PMCID: PMC5121015
- DOI: 10.1016/j.jaut.2016.08.002
Medullary thymic epithelial cells and CD8α+ dendritic cells coordinately regulate central tolerance but CD8α+ cells are dispensable for thymic regulatory T cell production
Abstract
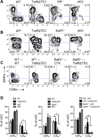
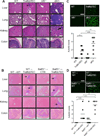
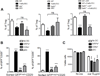
In the thymus, antigen presenting cells (APCs) namely, medullary thymic epithelial cells (mTECs) and thymic dendritic cells (tDCs) regulate T cell tolerance through elimination of autoreactive T cells and production of thymic T regulatory (tTreg) cells. How the different APCs in the thymus share the burden of tolerazing the emerging T cell repertoire remains unclear. For example, while mutations that inhibit mTEC development or function associate with peripheral autoimmunity, the role of tDCs in organ-specific autoimmunity and tTreg cell production remains controversial. In this report we used mice depleted of mTECs and/or CD8α+ DCs, to examine the contributions of these cell populations in thymic tolerance. We found that while mice depleted of CD8α+ DCs or mTECs were normal or developed liver inflammation respectively, combined depletion of mTECs and CD8α+ DCs resulted in overt peripheral autoimmunity. The autoimmune manifestations in mice depleted of both mTECs and CD8α+ cDCs associated with increased percentages of CD4+ and CD8+ T cells in the thymus. In contrast, while mTEC depletion resulted in reduced percentages of tTreg cells, no additional effect was observed when CD8α+ DCs were also depleted. These results reveal that: 1) mTECs and CD8α+ DCs cooperatively safeguard against peripheral autoimmunity through thymic T cell deletion; 2) CD8α+ DCs are dispensable for tTreg cell production, whereas mTECs play a non-redundant role in this process; 3) mTECs and CD8α+ DCs make unique contributions to tolerance induction that cannot be compensated for by other thymic APCs such as migratory SIRPα+ or plasmacytoid DCs.
Keywords: Autoimmunity; Dendritic cells; Medullary thymic epithelial cells; Regulatory T cells.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Transfer of Cell-Surface Antigens by Scavenger Receptor CD36 Promotes Thymic Regulatory T Cell Receptor Repertoire Development and Allo-tolerance.Immunity. 2018 May 15;48(5):923-936.e4. doi: 10.1016/j.immuni.2018.04.007. Epub 2018 May 8. Immunity. 2018. PMID: 29752065 Free PMC article.
-
Autoimmune-Mediated Thymic Atrophy Is Accelerated but Reversible in RelB-Deficient Mice.Front Immunol. 2018 May 22;9:1092. doi: 10.3389/fimmu.2018.01092. eCollection 2018. Front Immunol. 2018. PMID: 29872433 Free PMC article.
-
Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner.J Immunol. 2009 Sep 1;183(5):3053-63. doi: 10.4049/jimmunol.0900438. Epub 2009 Aug 12. J Immunol. 2009. PMID: 19675159
-
Medullary thymic epithelial cells, the indispensable player in central tolerance.Sci China Life Sci. 2013 May;56(5):392-8. doi: 10.1007/s11427-013-4482-4. Epub 2013 May 1. Sci China Life Sci. 2013. PMID: 23633070 Review.
-
Genetic basis of altered central tolerance and autoimmune diseases: a lesson from AIRE mutations.Int Rev Immunol. 2012 Oct;31(5):344-62. doi: 10.3109/08830185.2012.697230. Int Rev Immunol. 2012. PMID: 23083345 Review.
Cited by
-
Live-cell imaging reveals the relative contributions of antigen-presenting cell subsets to thymic central tolerance.Nat Commun. 2019 May 17;10(1):2220. doi: 10.1038/s41467-019-09727-4. Nat Commun. 2019. PMID: 31101805 Free PMC article.
-
Mechanisms of Direct and Indirect Presentation of Self-Antigens in the Thymus.Front Immunol. 2022 Jun 14;13:926625. doi: 10.3389/fimmu.2022.926625. eCollection 2022. Front Immunol. 2022. PMID: 35774801 Free PMC article. Review.
-
Formation of the Intrathymic Dendritic Cell Pool Requires CCL21-Mediated Recruitment of CCR7+ Progenitors to the Thymus.J Immunol. 2018 Jul 15;201(2):516-523. doi: 10.4049/jimmunol.1800348. Epub 2018 May 21. J Immunol. 2018. PMID: 29784760 Free PMC article.
-
Evaluating in vivo approaches for studying the roles of thymic DCs in T cell development in mice.Front Immunol. 2024 Aug 6;15:1451974. doi: 10.3389/fimmu.2024.1451974. eCollection 2024. Front Immunol. 2024. PMID: 39165362 Free PMC article. Review.
-
Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development.Front Nutr. 2022 Apr 20;9:878382. doi: 10.3389/fnut.2022.878382. eCollection 2022. Front Nutr. 2022. PMID: 35529463 Free PMC article. Review.
References
-
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. - PubMed
-
- Dresch C, Leverrier Y, Marvel J, Shortman K. Development of antigen cross-presentation capacity in dendritic cells. Trends in immunology. 2012;33:381–388. - PubMed
-
- Proietto AI, van Dommelen S, Wu L. The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunology and cell biology. 2009;87:39–45. - PubMed
-
- Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

