Complement-induced activation of the cardiac NLRP3 inflammasome in sepsis
- PMID: 27543123
- PMCID: PMC5102118
- DOI: 10.1096/fj.201600728R
Complement-induced activation of the cardiac NLRP3 inflammasome in sepsis
Abstract
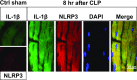
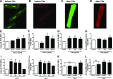
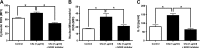
Cardiac dysfunction develops during sepsis in humans and rodents. In the model of polymicrobial sepsis induced by cecal ligation and puncture (CLP), we investigated the role of the NLRP3 inflammasome in the heart. Mouse heart homogenates from sham-procedure mice contained high mRNA levels of NLRP3 and IL-1β. Using the inflammasome protocol, exposure of cardiomyocytes (CMs) to LPS followed by ATP or nigericin caused release of mature IL-1β. Immunostaining of left ventricular frozen sections before and 8 h after CLP revealed the presence of NLRP3 and IL-1β proteins in CMs. CLP caused substantial increases in mRNAs for IL-1β and NLRP3 in CMs which are reduced in the absence of either C5aR1 or C5aR2. After CLP, NLRP3-/- mice showed reduced plasma levels of IL-1β and IL-6. In vitro exposure of wild-type CMs to recombinant C5a (rC5a) caused elevations in both cytosolic and nuclear/mitochondrial reactive oxygen species (ROS), which were C5a-receptor dependent. Use of a selective NOX2 inhibitor prevented increased cytosolic and nuclear/mitochondrial ROS levels and release of IL-1β. Finally, NLRP3-/- mice had reduced defects in echo/Doppler parameters in heart after CLP. These studies establish that the NLRP3 inflammasome contributes to the cardiomyopathy of polymicrobial sepsis.-Kalbitz, M., Fattahi, F., Grailer, J. J., Jajou, L., Malan, E. A., Zetoune, F. S., Huber-Lang, M., Russell, M. W., Ward, P. A. Complement-induced activation of the cardiac NLRP3 inflammasome in sepsis.
Keywords: C5a; C5a receptors; CLP; IL-1β.
© FASEB.
Figures

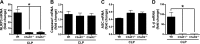

References
-
- Kalbitz M., Grailer J. J., Fattahi F., Jajou L., Herron T. J., Campbell K. F., Zetoune F. S., Bosmann M., Sarma J. V., Huber-Lang M., Gebhard F., Loaiza R., Valdivia H. H., Jalife J., Russell M. W., Ward P. A. (2015) Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 29, 2185–2193 - PMC - PubMed
-
- Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 - PubMed
-
- Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

