Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells
- PMID: 27543265
- PMCID: PMC5873581
- DOI: 10.1016/j.mce.2016.08.025
Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells
Abstract
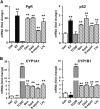
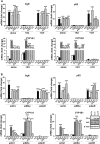
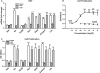
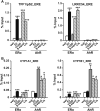
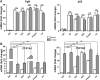
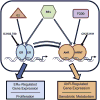
Botanical estrogen (BE) dietary supplements are consumed by women as substitutes for loss of endogenous estrogens at menopause. To examine the roles of estrogen receptor α (ERα) and aryl hydrocarbon receptor (AhR) and their crosstalk in the actions of BEs, we studied gene regulation and proliferation responses to four widely used BEs, genistein, daidzein, and S-equol from soy, and liquiritigen from licorice root in breast cancer and liver cells. BEs and estradiol (E2), acting through ERα, stimulated proliferation, ERα chromatin binding and target-gene expression. BEs but not E2, acting through AhR, bound to xenobiotic response element-containing chromatin sites and enhanced AhR target-gene expression (CYP1A1, CYP1B1). While E2 and TCDD acted quite selectively through their respective receptors, BEs acted via both receptors, with their AhR activity moderated by negative crosstalk through ERα. Both ERα and AhR should be considered as mediators of the biology and pharmacology of BEs.
Keywords: Aryl hydrocarbon receptor; Botanical estrogens; Cell proliferation; Estrogen receptor; Gene regulation; Xenobiotic metabolism.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures


Similar articles
-
Dioxin and estrogen signaling in lung adenocarcinoma cells with different aryl hydrocarbon receptor/estrogen receptor α phenotypes.Am J Respir Cell Mol Biol. 2013 Dec;49(6):1064-73. doi: 10.1165/rcmb.2012-0497OC. Am J Respir Cell Mol Biol. 2013. PMID: 23855798 Free PMC article.
-
12-O-tetradecanoylphorbol-13-acetate upregulates the Ah receptor and differentially alters CYP1B1 and CYP1A1 expression in MCF-7 breast cancer cells.J Cell Biochem. 1998 Sep 1;70(3):289-96. J Cell Biochem. 1998. PMID: 9706865
-
Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells.Cancer Lett. 2010 Dec 28;299(2):119-29. doi: 10.1016/j.canlet.2010.08.010. Epub 2010 Sep 16. Cancer Lett. 2010. PMID: 20846786 Free PMC article.
-
Cytochrome P450 1 family and cancers.J Steroid Biochem Mol Biol. 2015 Mar;147:24-30. doi: 10.1016/j.jsbmb.2014.11.003. Epub 2014 Nov 6. J Steroid Biochem Mol Biol. 2015. PMID: 25448748 Review.
-
Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells.J Mammary Gland Biol Neoplasia. 2000 Jul;5(3):295-306. doi: 10.1023/a:1009550912337. J Mammary Gland Biol Neoplasia. 2000. PMID: 14973392 Review.
Cited by
-
Can intra-articular daidzein injection reduce oxidative damage and early osteoarthritis in a rabbit temporomandibular joint model?BMC Oral Health. 2024 Oct 8;24(1):1193. doi: 10.1186/s12903-024-04990-4. BMC Oral Health. 2024. PMID: 39379866 Free PMC article.
-
Multi-Anticancer Activities of Phytoestrogens in Human Osteosarcoma.Int J Mol Sci. 2023 Aug 28;24(17):13344. doi: 10.3390/ijms241713344. Int J Mol Sci. 2023. PMID: 37686148 Free PMC article. Review.
-
Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts.Int J Mol Sci. 2021 Aug 16;22(16):8798. doi: 10.3390/ijms22168798. Int J Mol Sci. 2021. PMID: 34445499 Free PMC article. Review.
-
Menopause Hot Flashes and Molecular Mechanisms Modulated by Food-Derived Nutrients.Nutrients. 2024 Feb 26;16(5):655. doi: 10.3390/nu16050655. Nutrients. 2024. PMID: 38474783 Free PMC article. Review.
-
Aryl hydrocarbon receptor: An emerging player in breast cancer pathogenesis and its potential as a drug target (Review).Mol Med Rep. 2024 Jan;29(1):11. doi: 10.3892/mmr.2023.13134. Epub 2023 Nov 24. Mol Med Rep. 2024. PMID: 37997818 Free PMC article. Review.
References
-
- Ahmed S, Al-Saigh S, Matthews J. FOXA1 is essential for aryl hydrocarbon receptor-dependent regulation of cyclin G2. Mol. Cancer Res. 2012;10:636–648. - PubMed
-
- Beischlag TV, Perdew GH. ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 2005;280:21607–21611. - PubMed
-
- Bialesova L, Novotna A, Macejova D, Brtko J, Dvorak Z. Agonistic effect of selected isoflavones on arylhydrocarbon receptor in a novel AZ-AhR transgenic gene reporter human cell line. Gen. Physiol. Biophys. 2015;34:331–334. - PubMed
-
- Boonmuen N, Gong P, Ali Z, Chittiboyina AG, Khan I, Doerge DR, Helferich WG, Carlson KE, Martin T, Piyachaturawat P, Katzenellenbogen JA, Katzenellenbogen BS. Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities. Steroids. 2016;105:42–49. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

