Intron retention resulting from a silent mutation in the VWF gene that structurally influences the 5' splice site
- PMID: 27543438
- PMCID: PMC5161009
- DOI: 10.1182/blood-2016-02-699686
Intron retention resulting from a silent mutation in the VWF gene that structurally influences the 5' splice site
Abstract
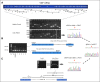
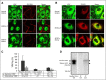
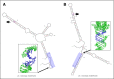
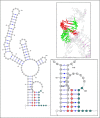
Disease-associated silent mutations are considered to affect the accurate pre-messenger RNA (mRNA) splicing either by influencing regulatory elements, leading to exon skipping, or by creating a new cryptic splice site. This study describes a new molecular pathological mechanism by which a silent mutation inhibits splicing and leads to intron retention. We identified a heterozygous silent mutation, c.7464C>T, in exon 44 of the von Willebrand factor (VWF) gene in a family with type 1 von Willebrand disease. In vivo and ex vivo transcript analysis revealed an aberrantly spliced transcript, with intron 44 retained in the mRNA, implying disruption of the first catalytic step of splicing at the 5' splice site (5'ss). The abnormal transcript with the retained intronic region coded a truncated protein that lacked the carboxy-terminal end of the VWF protein. Confocal immunofluorescence characterizations of blood outgrowth endothelial cells derived from the patient confirmed the presence of the truncated protein by demonstrating accumulation of VWF in the endoplasmic reticulum. In silico pre-mRNA secondary and tertiary structure analysis revealed that this substitution, despite its distal position from the 5'ss (85 bp downstream), induces cis alterations in pre-mRNA structure that result in the formation of a stable hairpin at the 5'ss. This hairpin sequesters the 5'ss residues involved in U1 small nuclear RNA interactions, thereby inhibiting excision of the pre-mRNA intronic region. This study is the first to show the allosteric-like/far-reaching effect of an exonic variation on pre-mRNA splicing that is mediated by structural changes in the pre-mRNA.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
Silent disruption: aberrant splicing in VWD.Blood. 2016 Oct 27;128(17):2113-2114. doi: 10.1182/blood-2016-09-735712. Blood. 2016. PMID: 27789437 No abstract available.
Similar articles
-
A Deep Exon Cryptic Splice Site Promotes Aberrant Intron Retention in a Von Willebrand Disease Patient.Int J Mol Sci. 2021 Dec 9;22(24):13248. doi: 10.3390/ijms222413248. Int J Mol Sci. 2021. PMID: 34948044 Free PMC article.
-
Characterization of aberrant splicing of von Willebrand factor in von Willebrand disease: an underrecognized mechanism.Blood. 2016 Jul 28;128(4):584-93. doi: 10.1182/blood-2015-10-678052. Epub 2016 Jun 17. Blood. 2016. PMID: 27317792 Free PMC article. Clinical Trial.
-
High-throughput analysis revealed mutations' diverging effects on SMN1 exon 7 splicing.RNA Biol. 2019 Oct;16(10):1364-1376. doi: 10.1080/15476286.2019.1630796. Epub 2019 Jun 19. RNA Biol. 2019. PMID: 31213135 Free PMC article.
-
Pick one, but be quick: 5' splice sites and the problems of too many choices.Genes Dev. 2013 Jan 15;27(2):129-44. doi: 10.1101/gad.209759.112. Genes Dev. 2013. PMID: 23348838 Free PMC article. Review.
-
A novel role of U1 snRNP: Splice site selection from a distance.Biochim Biophys Acta Gene Regul Mech. 2019 Jun;1862(6):634-642. doi: 10.1016/j.bbagrm.2019.04.004. Epub 2019 Apr 28. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 31042550 Free PMC article. Review.
Cited by
-
A Homozygous Deep Intronic Variant Causes Von Willebrand Factor Deficiency and Lack of Endothelial-Specific Secretory Organelles, Weibel-Palade Bodies.Int J Mol Sci. 2022 Mar 13;23(6):3095. doi: 10.3390/ijms23063095. Int J Mol Sci. 2022. PMID: 35328514 Free PMC article.
-
Multifaceted pathomolecular mechanism of a VWF large deletion involved in the pathogenesis of severe VWD.Blood Adv. 2022 Feb 8;6(3):1038-1053. doi: 10.1182/bloodadvances.2021005895. Blood Adv. 2022. PMID: 34861678 Free PMC article.
-
A Novel Synonymous Variant of PAX2 in Monochorionic Diamniotic Twins With Bilateral Renal Agenesis: A Case Report and Literature Review.Mol Genet Genomic Med. 2025 Jun;13(6):e70113. doi: 10.1002/mgg3.70113. Mol Genet Genomic Med. 2025. PMID: 40515469 Free PMC article. Review.
-
Unraveling the effect of silent, intronic and missense mutations on VWF splicing: contribution of next generation sequencing in the study of mRNA.Haematologica. 2019 Mar;104(3):587-598. doi: 10.3324/haematol.2018.203166. Epub 2018 Oct 25. Haematologica. 2019. PMID: 30361419 Free PMC article.
-
A Deep Exon Cryptic Splice Site Promotes Aberrant Intron Retention in a Von Willebrand Disease Patient.Int J Mol Sci. 2021 Dec 9;22(24):13248. doi: 10.3390/ijms222413248. Int J Mol Sci. 2021. PMID: 34948044 Free PMC article.
References
-
- Lyons SE, Ginsburg D. Molecular and cellular biology of von Willebrand factor. Trends Cardiovasc Med. 1994;4(1):34–39. - PubMed
-
- Goodeve AC. The genetic basis of von Willebrand disease. Blood Rev. 2010;24(3):123–134. - PubMed
-
- Federici AB. Classification of inherited von Willebrand disease and implications in clinical practice. Thromb Res. 2009;124(suppl 1):S2–S6. - PubMed
-
- Berber E, James PD, Hough C, Lillicrap D. An assessment of the pathogenic significance of the R924Q von Willebrand factor substitution. J Thromb Haemost. 2009;7(10):1672–1679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

