Resolvin E1 Reverses Experimental Periodontitis and Dysbiosis
- PMID: 27543615
- PMCID: PMC5026932
- DOI: 10.4049/jimmunol.1600859
Resolvin E1 Reverses Experimental Periodontitis and Dysbiosis
Abstract
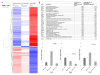
Periodontitis is a biofilm-induced inflammatory disease characterized by dysbiosis of the commensal periodontal microbiota. It is unclear how natural regulation of inflammation affects the periodontal biofilm. Promoters of active resolution of inflammation, including resolvin E1 (RvE1), effectively treat inflammatory periodontitis in animal models. The goals of this study were 1) to compare periodontal tissue gene expression in different clinical conditions, 2) to determine the impact of local inflammation on the composition of subgingival bacteria, and 3) to understand how inflammation impacts these changes. Two clinically relevant experiments were performed in rats: prevention and treatment of ligature-induced periodontitis with RvE1 topical treatment. The gingival transcriptome was evaluated by RNA sequencing of mRNA. The composition of the subgingival microbiota was characterized by 16S rDNA sequencing. Periodontitis was assessed by bone morphometric measurements and histomorphometry of block sections. H&E and tartrate-resistant acid phosphatase staining were used to characterize and quantify inflammatory changes. RvE1 treatment prevented bone loss in ligature-induced periodontitis. Osteoclast density and inflammatory cell infiltration in the RvE1 groups were lower than those in the placebo group. RvE1 treatment reduced expression of inflammation-related genes, returning the expression profile to one more similar to health. Treatment of established periodontitis with RvE1 reversed bone loss, reversed inflammatory gene expression, and reduced osteoclast density. Assessment of the rat subgingival microbiota after RvE1 treatment revealed marked changes in both prevention and treatment experiments. The data suggest that modulation of local inflammation has a major role in shaping the composition of the subgingival microbiota.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

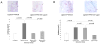


References
-
- Heitz-Mayfield LJ, Lang NP. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontology 2000. 2013;62:218–231. - PubMed
-
- Slots J. Selection of antimicrobial agents in periodontal therapy. Journal of periodontal research. 2002;37:389–398. - PubMed
-
- Haffajee AD, Socransky SS. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontology 2000. 2006;42:7–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

