ABT-719 for the Prevention of Acute Kidney Injury in Patients Undergoing High-Risk Cardiac Surgery: A Randomized Phase 2b Clinical Trial
- PMID: 27543797
- PMCID: PMC5015281
- DOI: 10.1161/JAHA.116.003549
ABT-719 for the Prevention of Acute Kidney Injury in Patients Undergoing High-Risk Cardiac Surgery: A Randomized Phase 2b Clinical Trial
Abstract
Background: Patients undergoing cardiac surgeries with cardiopulmonary bypass (on-pump) have a high risk for acute kidney injury (AKI). We tested ABT-719, a novel α-melanocyte-stimulating hormone analog, for prevention of AKI in postoperative cardiac surgery patients.
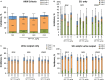
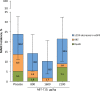
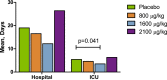
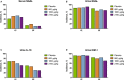
Methods and results: This phase 2b randomized, double-blind, placebo-controlled trial included adult patients with stable renal function undergoing high-risk on-pump cardiac surgery in the United States and Denmark. Participants received placebo (n=61) or cumulative ABT-719 doses of 800 (n=59), 1600 (n=61), or 2100 μg/kg (n=59). Primary outcome was development of AKI based on Acute Kidney Injury Network (AKIN) criteria, measured utilizing preoperative creatinine value and maximum value within 48 hours and urine output within the first 42 hours postsurgery. Secondary outcomes included incidence of AKI based on maximal changes from baseline in novel AKI biomarkers over a 72-hour period after clamp release and length of intensive care unit stays through 90 days postsurgery. A total of 65.5%, 62.7%, and 69.6% of patients in the 800-, 1600-, and 2100-μg/kg groups, respectively, developed AKI (stages 1, 2, and 3 combined) versus 65.5% in the placebo group (for each pair-wise comparison with placebo, P=0.966, 0.815, and 0.605, respectively). Adverse events occurred at a similar rate in all treatment groups.
Conclusions: ABT-719 treatment did not lower AKI incidence using AKIN criteria, influence the elevations of novel biomarkers, or change 90-day outcomes in patients after cardiac surgery.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01777165.
Keywords: acute kidney injury; cardiopulmonary bypass; clinical trial; kidney; renal; renal function; α‐melanocyte‐stimulating hormone.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
References
-
- Dasta JF, Kane‐Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. - PubMed
-
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. - PubMed
-
- Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552–2558. - PubMed
-
- Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–1547. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

