Structural basis of selectivity and neutralizing activity of a TGFα/epiregulin specific antibody
- PMID: 27543934
- PMCID: PMC5079263
- DOI: 10.1002/pro.3023
Structural basis of selectivity and neutralizing activity of a TGFα/epiregulin specific antibody
Abstract
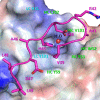
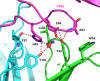
Recent studies have implicated a role of the epidermal growth factor receptor (EGFR) pathway in kidney disease. Skin toxicity associated with therapeutics which completely block the EGFR pathway precludes their use in chronic dosing. Therefore, we developed antibodies which specifically neutralize the EGFR ligands TGFα (transforming growth factor-alpha) and epiregulin but not EGF (epidermal growth factor), amphiregulin, betacellulin, HB-EGF (heparin-binding epidermal growth factor), or epigen. The epitope of one such neutralizing antibody, LY3016859, was characterized in detail to elucidate the structural basis for ligand specificity. Here we report a crystal structure of the LY3016859 Fab fragment in complex with soluble human TGFα. Our data demonstrate a conformational epitope located primarily within the C-terminal subdomain of the ligand. In addition, point mutagenesis experiments were used to highlight specific amino acids which are critical for both antigen binding and neutralization, most notably Ala41 , Glu44 , and His45 . These results illustrate the structural basis for the ligand specificity/selectivity of LY3016859 and could also provide insight into further engineering to alter specificity and/or affinity of LY3016859.
Keywords: X-ray crystallography; epiregulin; epitope mapping; kidney disease; transforming growth factor alpha.
© 2016 The Protein Society.
Figures

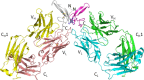
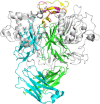
Similar articles
-
Generation and activity of a humanized monoclonal antibody that selectively neutralizes the epidermal growth factor receptor ligands transforming growth factor-α and epiregulin.J Pharmacol Exp Ther. 2014 May;349(2):330-43. doi: 10.1124/jpet.113.210765. Epub 2014 Feb 11. J Pharmacol Exp Ther. 2014. PMID: 24518034
-
The significance of valine 33 as a ligand-specific epitope of transforming growth factor alpha.J Biol Chem. 1996 Jun 28;271(26):15367-72. doi: 10.1074/jbc.271.26.15367. J Biol Chem. 1996. PMID: 8663070
-
Epiregulin is critical for the acinar cell regeneration of the submandibular gland in a mouse duct ligation model.J Oral Pathol Med. 2014 May;43(5):378-87. doi: 10.1111/jop.12145. Epub 2013 Dec 20. J Oral Pathol Med. 2014. PMID: 24354788
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
-
EGF receptor ligands: recent advances.F1000Res. 2016 Sep 8;5:F1000 Faculty Rev-2270. doi: 10.12688/f1000research.9025.1. eCollection 2016. F1000Res. 2016. PMID: 27635238 Free PMC article. Review.
Cited by
-
Design and Evolution of Enhanced Peptide-Peptide Ligation for Modular Transglutaminase Assembly.Bioconjug Chem. 2023 Jun 21;34(6):1019-1036. doi: 10.1021/acs.bioconjchem.3c00122. Epub 2023 Jun 8. Bioconjug Chem. 2023. PMID: 37289810 Free PMC article.
-
Mobile Affinity Sorbent Chromatography.Anal Chem. 2018 Feb 6;90(3):1668-1676. doi: 10.1021/acs.analchem.7b03117. Epub 2018 Jan 9. Anal Chem. 2018. PMID: 29260867 Free PMC article.
-
Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage.Mediators Inflamm. 2018 Dec 23;2018:8739473. doi: 10.1155/2018/8739473. eCollection 2018. Mediators Inflamm. 2018. PMID: 30670929 Free PMC article. Review.
References
-
- Schlessinger J (2002) Ligand‐induced, receptor‐mediated dimerization and activation of EGF receptor. Cell 110:669–672. - PubMed
-
- Carpenter G (1983) The biochemistry and physiology of the receptor‐kinase for epidermal growth factor. Mol Cell Endocrinol 31:1–19. - PubMed
-
- Schlessinger J, Schreiber AB, Levi A, Lax I, Libermann T, Yarden Y (1983) Regulation of cell proliferation by epidermal growth factor. CRC Crit Rev Biochem 14:93–111. - PubMed
-
- Harris RC, Chung E, Coffey RJ (2003) EGF receptor ligands. Exp Cell Res 284:2–13. - PubMed
-
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, Jorissen RN, Nice EC, Burgess AW, Ward CW (2002) Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell 110:763–773. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

