Tumor cells with neuronal intermediate progenitor features define a subgroup of 1p/19q co-deleted anaplastic gliomas
- PMID: 27543943
- PMCID: PMC8029323
- DOI: 10.1111/bpa.12434
Tumor cells with neuronal intermediate progenitor features define a subgroup of 1p/19q co-deleted anaplastic gliomas
Abstract
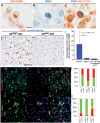
The integrated diagnosis of anaplastic oligodendroglioma, IDH mutant and 1p/19q co-deleted, grade III (O3id ) is a histomolecular entity that WHO 2016 classification distinguished from other diffuse gliomas by specific molecular alterations. In contrast, its cell portrait is less well known. The present study is focused on intertumor and intratumor, cell lineage-oriented, heterogeneity in O3id . Based on pathological, transcriptomic and immunophenotypic studies, a novel subgroup of newly diagnosed O3id overexpressing neuronal intermediate progenitor (NIP) genes was identified. This NIP overexpression pattern in O3id is associated with: (i) morphological and immunohistochemical similarities with embryonic subventricular zone, (ii) proliferating tumor cell subpopulation with NIP features including expression of INSM1 and no expression of SOX9, (iii) mutations in critical genes involved in NIP biology and, (iv) increased tumor necrosis. Interestingly, NIP tumor cell subpopulation increases in O3id recurrence compared with paired newly diagnosed tumors. Our results, validated in an independent cohort, emphasize intertumor and intratumor heterogeneity in O3id and identified a tumor cell subpopulation exhibiting NIP characteristics that is potentially critical in oncogenesis of O3id . A better understanding of spatial and temporal intratumor cell heterogeneity in O3id will open new therapeutic avenues overcoming resistance to current antitumor treatments.
Keywords: 1p/19q co-deletion; anaplastic oligodendroglioma; embryonic subventricular zone; neuronal intermediate progenitor.
© 2016 International Society of Neuropathology.
Figures



References
-
- Alentorn A, Sanson M, Idbaih A (2012) Oligodendrogliomas: new insights from the genetics and perspectives. Curr Opin Oncol 24:687–693. - PubMed
-
- Bannykh SI, Stolt CC, Kim J, Perry A, Wegner M (2006) Oligodendroglial‐specific transcriptional factor SOX10 is ubiquitously expressed in human gliomas. J Neurooncol 76:115–127. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

