Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain
- PMID: 27544482
- PMCID: PMC5102759
- DOI: 10.1016/j.nbd.2016.08.005
Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain
Abstract
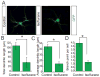
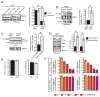
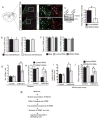
Anesthetics including isoflurane are known to induce neuronal dysfunction in the developing brain, however, the underlying mechanism is mostly unknown. The transcriptional activation of CREB (cyclic AMP response element binding protein) and the alterations in acetylation of histones modulated by several histone deacetylases such as HDAC4 (histone deacetylase 4) are known to contribute to synaptic plasticity in the brain. Here we have shown that administration of isoflurane (1.4%) for 2h leads to transcriptional inactivation of CREB which results in loss of dendritic outgrowth and decreased expression level of proteins essential for memory and cognitive functions, such as BDNF, and c-fos in the developing brain of mice at postnatal day 7 (PND7). To elucidate the molecular mechanism, we found that exposure to isoflurane leads to an increase in nuclear translocation of HDAC4, which interacts with CREB in the nucleus. This event, in turn, results in a decrease in interaction between an acetyltransferase, CBP, and CREB that ultimately leads to transcriptional inactivation of CREB. As a result, the expression level of BDNF, and c-fos were significantly down-regulated after administration of isoflurane in PND7 brain. Depletion of HDAC4 in PND7 brain rescues the transcriptional activation of CREB along with augmentation in the level of the expression level of BDNF and c-fos. Moreover, administration of lentiviral particles of HDAC4 RNAi in primary neurons rescues neurite outgrowth following isoflurane treatment. Taken together, our study suggests that HDAC4-induced transcriptional inactivation of CREB is responsible for isoflurane-induced cognitive dysfunction in the brain.
Keywords: CREB; HDAC; Isoflurane; Synapse.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



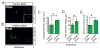
Comment in
-
Anesthetics and epigenetics.Oncotarget. 2016 Nov 29;7(48):78220-78221. doi: 10.18632/oncotarget.13143. Oncotarget. 2016. PMID: 27829240 Free PMC article. No abstract available.
References
-
- Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

