Filarial Infection or Antigen Administration Improves Glucose Tolerance in Diet-Induced Obese Mice
- PMID: 27544668
- PMCID: PMC6743339
- DOI: 10.1159/000448401
Filarial Infection or Antigen Administration Improves Glucose Tolerance in Diet-Induced Obese Mice
Abstract
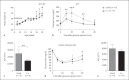
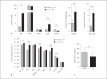
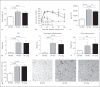
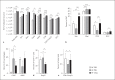
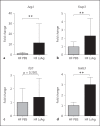
Helminths induce type 2 immune responses and establish an anti-inflammatory milieu in their hosts. This immunomodulation was previously shown to improve diet-induced insulin resistance which is linked to chronic inflammation. In the current study, we demonstrate that infection with the filarial nematode Litomosoides sigmodontis increased the eosinophil number and alternatively activated macrophage abundance within epididymal adipose tissue (EAT) and improved glucose tolerance in diet-induced obese mice in an eosinophil-dependent manner. L. sigmodontis antigen (LsAg) administration neither altered the body weight of animals nor adipose tissue mass or adipocyte size, but it triggered type 2 immune responses, eosinophils, alternatively activated macrophages, and type 2 innate lymphoid cells in EAT. Improvement in glucose tolerance by LsAg treatment remained even in the absence of Foxp3+ regulatory T cells. Furthermore, PCR array results revealed that LsAg treatment reduced inflammatory immune responses and increased the expression of genes related to insulin signaling (Glut4, Pde3b, Pik3r1, and Hk2) and fatty acid uptake (Fabp4 and Lpl). Our investigation demonstrates that L. sigmodontis infection and LsAg administration reduce diet-induced EAT inflammation and improve glucose tolerance. Helminth-derived products may, therefore, offer new options to improve insulin sensitivity, while loss of helminth infections in developing and developed countries may contribute to the recent increase in the prevalence of type 2 diabetes.
© 2016 S. Karger AG, Basel.
Conflict of interest statement
There are no competing financial interests.
Figures



References
-
- International Diabetes Federation IDF Diabetes Atlas, ed 6, 2014. Brussels, International Diabetes Federation. 2014 http://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf.
-
- Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23:431–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

