Amyloid precursor protein processing and bioenergetics
- PMID: 27545490
- PMCID: PMC5316384
- DOI: 10.1016/j.brainresbull.2016.08.009
Amyloid precursor protein processing and bioenergetics
Abstract
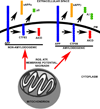
The processing of amyloid precursor protein (APP) to amyloid beta (Aβ) is of great interest to the Alzheimer's disease (AD) field. Decades of research define how APP is altered to form Aβ, and how Aβ generates oligomers, protofibrils, and fibrils. Numerous signaling pathways and changes in cell physiology are known to influence APP processing. Existing data additionally indicate a relationship exists between mitochondria, bioenergetics, and APP processing. Here, we review data that address whether mitochondrial function and bioenergetics modify APP processing and Aβ production.
Keywords: Alpha secretase; Alzheimer's disease; Amyloid beta; Amyloid precursor protein; BACE1; Bioenergetics; Gamma secretase; Mitochondria.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Aho L, et al. Immunohistochemical visualization of amyloid-beta protein precursor and amyloid-beta in extra- and intracellular compartments in the human brain. J Alzheimers Dis. 2010;20:1015–1028. - PubMed
-
- Allinson TM, et al. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Asai M, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003;301:231–235. - PubMed
-
- Barrientos A, et al. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52:284–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

