Retention of Native Protein Structures in the Absence of Solvent: A Coupled Ion Mobility and Spectroscopic Study
- PMID: 27545682
- PMCID: PMC5113788
- DOI: 10.1002/anie.201606029
Retention of Native Protein Structures in the Absence of Solvent: A Coupled Ion Mobility and Spectroscopic Study
Abstract
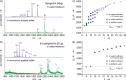
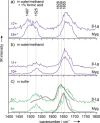
Can the structures of small to medium-sized proteins be conserved after transfer from the solution phase to the gas phase? A large number of studies have been devoted to this topic, however the answer has not been unambiguously determined to date. A clarification of this problem is important since it would allow very sensitive native mass spectrometry techniques to be used to address problems relevant to structural biology. A combination of ion-mobility mass spectrometry with infrared spectroscopy was used to investigate the secondary and tertiary structure of proteins carefully transferred from solution to the gas phase. The two proteins investigated are myoglobin and β-lactoglobulin, which are prototypical examples of helical and β-sheet proteins, respectively. The results show that for low charge states under gentle conditions, aspects of the native secondary and tertiary structure can be conserved.
Keywords: IR spectroscopy; gas-phase reactions; mass spectrometry; protein folding; protein structures.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
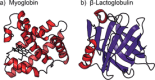
Similar articles
-
Protonation State-Induced Unfolding of Protein Secondary Structure in the Gas Phase.J Phys Chem Lett. 2024 Sep 19;15(37):9374-9379. doi: 10.1021/acs.jpclett.4c02103. Epub 2024 Sep 6. J Phys Chem Lett. 2024. PMID: 39240543
-
Covalent Cross-Linking as an Enabler for Structural Mass Spectrometry.Anal Chem. 2019 Oct 15;91(20):12808-12818. doi: 10.1021/acs.analchem.9b02491. Epub 2019 Sep 30. Anal Chem. 2019. PMID: 31490660
-
The effects of solution additives and gas-phase modifiers on the molecular environment and conformational space of common heme proteins.Rapid Commun Mass Spectrom. 2019 Mar 15;33(5):399-404. doi: 10.1002/rcm.8347. Rapid Commun Mass Spectrom. 2019. PMID: 30421840
-
High-pressure NMR spectroscopy for characterizing folding intermediates and denatured states of proteins.Methods. 2004 Sep;34(1):133-43. doi: 10.1016/j.ymeth.2004.03.010. Methods. 2004. PMID: 15283922 Review.
-
Ion mobility mass spectrometry of proteins and protein assemblies.Chem Soc Rev. 2010 May;39(5):1633-55. doi: 10.1039/b914002f. Epub 2009 Nov 25. Chem Soc Rev. 2010. PMID: 20419213 Review.
Cited by
-
Epigallocatechin-3-gallate Inhibits Cu(II)-Induced β-2-Microglobulin Amyloid Formation by Binding to the Edge of Its β-Sheets.Biochemistry. 2020 Mar 17;59(10):1093-1103. doi: 10.1021/acs.biochem.0c00043. Epub 2020 Mar 3. Biochemistry. 2020. PMID: 32100530 Free PMC article.
-
Norovirus-glycan interactions - how strong are they really?Biochem Soc Trans. 2022 Feb 28;50(1):347-359. doi: 10.1042/BST20210526. Biochem Soc Trans. 2022. PMID: 34940787 Free PMC article.
-
Computational Insights into Compaction of Gas-Phase Protein and Protein Complex Ions in Native Ion Mobility-Mass Spectrometry.Trends Analyt Chem. 2019 Jul;116:282-291. doi: 10.1016/j.trac.2019.04.023. Epub 2019 Apr 30. Trends Analyt Chem. 2019. PMID: 31983791 Free PMC article.
-
The assessment of Pseudomonas aeruginosa lectin LecA binding characteristics of divalent galactosides using multiple techniques.Glycobiology. 2021 Dec 18;31(11):1490-1499. doi: 10.1093/glycob/cwab074. Glycobiology. 2021. PMID: 34255029 Free PMC article.
-
Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown 8/Protonated Serine Noncovalent Complexes in the Gas Phase vs in Solution: Quantum Chemical Analysis.ACS Omega. 2024 May 23;9(22):23793-23801. doi: 10.1021/acsomega.4c01782. eCollection 2024 Jun 4. ACS Omega. 2024. PMID: 38854571 Free PMC article.
References
-
- Pandey A., Mann M., Nature 2000, 405, 837–846. - PubMed
-
- None
-
- Heck A. J. R., Nat. Methods 2008, 5, 927–933; - PubMed
-
- Ruotolo B. T., Robinson C. V., Curr. Opin. Chem. Biol. 2006, 10, 402–408. - PubMed
-
- Siuzdak G., Bothner B., Yeager M., Brugidou C., Fauquet C. M., Hoey K., Chang C. M., Chem. Biol. 1996, 3, 45–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

