Singlet versus Triplet Reactivity in an Mn(V)-Oxo Species: Testing Theoretical Predictions Against Experimental Evidence
- PMID: 27545752
- PMCID: PMC5228574
- DOI: 10.1021/jacs.6b05027
Singlet versus Triplet Reactivity in an Mn(V)-Oxo Species: Testing Theoretical Predictions Against Experimental Evidence
Abstract
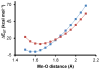
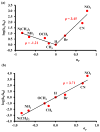
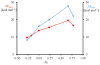
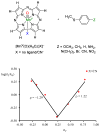
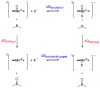
Discerning the factors that control the reactivity of high-valent metal-oxo species is critical to both an understanding of metalloenzyme reactivity and related transition metal catalysts. Computational studies have suggested that an excited higher spin state in a number of metal-oxo species can provide a lower energy barrier for oxidation reactions, leading to the conclusion that this unobserved higher spin state complex should be considered as the active oxidant. However, testing these computational predictions by experiment is difficult and has rarely been accomplished. Herein, we describe a detailed computational study on the role of spin state in the reactivity of a high-valent manganese(V)-oxo complex with para-Z-substituted thioanisoles and utilize experimental evidence to distinguish between the theoretical results. The calculations show an unusual change in mechanism occurs for the dominant singlet spin state that correlates with the electron-donating property of the para-Z substituent, while this change is not observed on the triplet spin state. Minimum energy crossing point calculations predict small spin-orbit coupling constants making the spin state change from low spin to high spin unlikely. The trends in reactivity for the para-Z-substituted thioanisole derivatives provide an experimental measure for the spin state reactivity in manganese-oxo corrolazine complexes. Hence, the calculations show that the V-shaped Hammett plot is reproduced by the singlet surface but not by the triplet state trend. The substituent effect is explained with valence bond models, which confirm a change from an electrophilic to a nucleophilic mechanism through a change of substituent.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

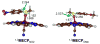

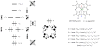
References
-
-
See, e.g.: Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235–349.Bugg TDH. Curr Opin Chem Biol. 2001;5:550–555.Ryle MJ, Hausinger RP. Curr Opin Chem Biol. 2002;6:193–201.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986.Abu-Omar MM, Loaiza A, Hontzeas N. Chem Rev. 2005;105:2227–2252.Bruijnincx PCM, van Koten G, Klein Gebbink RJM. Chem Soc Rev. 2008;37:2716–2744.Kryatov SV, Rybak-Akimova EV, Schindler S. Chem Rev. 2005;105:2175–2226.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

