Collagen I hydrogel microstructure and composition conjointly regulate vascular network formation
- PMID: 27545811
- PMCID: PMC5045803
- DOI: 10.1016/j.actbio.2016.08.028
Collagen I hydrogel microstructure and composition conjointly regulate vascular network formation
Abstract
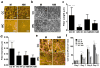
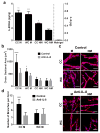
Neovascularization is a hallmark of physiological and pathological tissue remodeling that is regulated in part by the extracellular matrix (ECM). Collagen I hydrogels or Matrigel are frequently used to study vascular network formation; however, in isolation these materials do not typically mimic the integrated effects of ECM structure and composition that may influence endothelial cells in vivo. Here, we have utilized microfabricated 3D culture models to control collagen I microstructure in the presence and absence of Matrigel and tested the effect of these variations on vascular network formation by human cerebral microvascular endothelial cells (hCMECs). Varied collagen microarchitecture was achieved by adjusting the gelation temperature and subsequently confirmed by structural analysis. Casting at colder temperature increased collagen fiber thickness and length, and inclusion of Matrigel further pronounced these differences. Interestingly, the presence of Matrigel affected vascular network formation by modulating hCMEC growth, whereas altered collagen fiber structure impacted the morphology and maturity of the developed vascular network. These differences were related to substrate-dependent changes in interleukin-8 (IL-8) secretion and were functionally relevant as vascular networks preformed in more fibrillar, Matrigel-containing hydrogels promoted angiogenic sprouting. Our studies indicate that collagen hydrogel microstructure and composition conjointly regulate vascular network formation with implications for translational and basic science approaches.
Statement of significance: Neovascularization is a hallmark of both tissue homeostasis and disease and is in part regulated by cell remodeling that occurs in the extracellular matrix (ECM). The use of bio-mimetic hydrogel cell culture systems has been used to study the effects of the ECM on cell behavior. Here, we employ a hydrogel system that enables control over both the structure and composition of the ECM and subsequently investigated the effects that these have on blood vessel dynamics. Finally, we linked these differences to changes in protein secretion and the implications that this may play in scientific translation.
Keywords: Endothelial cells; Extracellular matrix; Interleukin-8; Microenvironment; Vascularization.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures




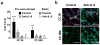
Similar articles
-
In vitro angiogenesis induced by tumor-endothelial cell co-culture in bilayered, collagen I hydrogel bioengineered tumors.Tissue Eng Part C Methods. 2013 Nov;19(11):864-74. doi: 10.1089/ten.TEC.2012.0684. Epub 2013 Apr 26. Tissue Eng Part C Methods. 2013. PMID: 23516987 Free PMC article.
-
Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis.Acta Biomater. 2018 Feb;67:270-281. doi: 10.1016/j.actbio.2017.11.046. Epub 2017 Dec 6. Acta Biomater. 2018. PMID: 29223704
-
Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks.J Tissue Eng Regen Med. 2011 Apr;5(4):e74-86. doi: 10.1002/term.389. Epub 2011 Jan 10. J Tissue Eng Regen Med. 2011. PMID: 21413157 Free PMC article.
-
Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications.Biotechnol Prog. 2017 May;33(3):580-589. doi: 10.1002/btpr.2457. Epub 2017 Mar 20. Biotechnol Prog. 2017. PMID: 28247962 Review.
-
How cells sense extracellular matrix stiffness: a material's perspective.Curr Opin Biotechnol. 2013 Oct;24(5):948-53. doi: 10.1016/j.copbio.2013.03.020. Epub 2013 Apr 20. Curr Opin Biotechnol. 2013. PMID: 23611564 Free PMC article. Review.
Cited by
-
Collagen microarchitecture mechanically controls myofibroblast differentiation.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11387-11398. doi: 10.1073/pnas.1919394117. Epub 2020 May 8. Proc Natl Acad Sci U S A. 2020. PMID: 32385149 Free PMC article.
-
Growth Factor-Free Vascularization of Marine-Origin Collagen Sponges Using Cryopreserved Stromal Vascular Fractions from Human Adipose Tissue.Mar Drugs. 2022 Sep 30;20(10):623. doi: 10.3390/md20100623. Mar Drugs. 2022. PMID: 36286447 Free PMC article.
-
Microvessels derived from hiPSCs are a novel source for angiogenesis and tissue regeneration.J Tissue Eng. 2022 Dec 26;13:20417314221143240. doi: 10.1177/20417314221143240. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 36600998 Free PMC article.
-
The importance of 3D fibre architecture in cancer and implications for biomaterial model design.Nat Rev Cancer. 2024 Jul;24(7):461-479. doi: 10.1038/s41568-024-00704-8. Epub 2024 Jun 17. Nat Rev Cancer. 2024. PMID: 38886573 Review.
-
Phototunable interpenetrating polymer network hydrogels to stimulate the vasculogenesis of stem cell-derived endothelial progenitors.Acta Biomater. 2021 Mar 1;122:133-144. doi: 10.1016/j.actbio.2020.12.041. Epub 2020 Dec 21. Acta Biomater. 2021. PMID: 33359297 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

