Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance
- PMID: 27545889
- PMCID: PMC5007173
- DOI: 10.1016/j.celrep.2016.07.075
Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance
Abstract
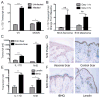
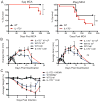
Cells undergoing xenobiotic or oxidative stress activate the transcription factor nuclear factor erythroid-derived 2-like 2 (Nrf2), which initiates an intrinsic "stress surveillance" pathway. We recently found that the cytokine IL-17D effects a form of extrinsic stress surveillance by inducing antitumor immunity, but how IL-17D is regulated remains unknown. Here, we show that Nrf2 induced IL-17D in cancer cell lines. Moreover, both Nrf2 and IL-17D were induced in primary tumors as well as during viral infection in vivo. Expression of IL-17D in tumors and virally infected cells is essential for optimal protection of the host as il17d(-/-) mice experienced a higher incidence of tumors and exacerbated viral infections compared to wild-type (WT) animals. Moreover, activating Nrf2 to induce IL-17D in established tumors led to natural killer cell-dependent tumor regression. These data demonstrate that Nrf2 can initiate both intrinsic and extrinsic stress surveillance pathways and highlight the use of Nrf2 agonists as immune therapies for cancer and infection.
Keywords: NK cells; Nrf2; immunosurveillance; innate immunity; interleukin-17D; tumor rejection.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures

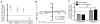


Similar articles
-
Interleukin-17D and Nrf2 mediate initial innate immune cell recruitment and restrict MCMV infection.Sci Rep. 2018 Sep 12;8(1):13670. doi: 10.1038/s41598-018-32011-2. Sci Rep. 2018. PMID: 30209334 Free PMC article.
-
The ancient cytokine IL-17D is regulated by Nrf2 and mediates tumor and virus surveillance.Cytokine. 2017 Mar;91:10-12. doi: 10.1016/j.cyto.2016.11.017. Epub 2016 Dec 9. Cytokine. 2017. PMID: 27940089 Free PMC article. Review.
-
Nuclear factor erythroid 2-related factor 2 potentiates the generation of inflammatory cytokines by intestinal epithelial cells during hyperoxia by inducing the expression of interleukin 17D.Toxicology. 2021 Jun 15;457:152820. doi: 10.1016/j.tox.2021.152820. Epub 2021 May 21. Toxicology. 2021. PMID: 34023435
-
Interleukin-17D mediates tumor rejection through recruitment of natural killer cells.Cell Rep. 2014 May 22;7(4):989-98. doi: 10.1016/j.celrep.2014.03.073. Epub 2014 May 1. Cell Rep. 2014. PMID: 24794441 Free PMC article.
-
Simultaneous Activation of Nrf2 and Elevation of Dietary and Endogenous Antioxidant Chemicals for Cancer Prevention in Humans.J Am Coll Nutr. 2016;35(2):175-84. doi: 10.1080/07315724.2014.1003419. Epub 2015 Jul 7. J Am Coll Nutr. 2016. PMID: 26151600 Review.
Cited by
-
Insights into potential mechanisms of asthma patients with COVID-19: A study based on the gene expression profiling of bronchoalveolar lavage fluid.Comput Biol Med. 2022 Jul;146:105601. doi: 10.1016/j.compbiomed.2022.105601. Epub 2022 May 19. Comput Biol Med. 2022. PMID: 35751199 Free PMC article.
-
Interleukin-17 in rheumatoid arthritis: Trials and tribulations.J Exp Med. 2020 Mar 2;217(3):e20192048. doi: 10.1084/jem.20192048. J Exp Med. 2020. PMID: 32023342 Free PMC article. Review.
-
Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications.Cold Spring Harb Perspect Biol. 2018 Apr 2;10(4):a028522. doi: 10.1101/cshperspect.a028522. Cold Spring Harb Perspect Biol. 2018. PMID: 28620097 Free PMC article. Review.
-
Health position paper and redox perspectives - Bench to bedside transition for pharmacological regulation of NRF2 in noncommunicable diseases.Redox Biol. 2025 Apr;81:103569. doi: 10.1016/j.redox.2025.103569. Epub 2025 Mar 3. Redox Biol. 2025. PMID: 40059038 Free PMC article. Review.
-
The Role of NRF2 in Trinucleotide Repeat Expansion Disorders.Antioxidants (Basel). 2024 May 26;13(6):649. doi: 10.3390/antiox13060649. Antioxidants (Basel). 2024. PMID: 38929088 Free PMC article. Review.
References
-
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a Tcell- dependent mechanism. Blood. 2002;99:2114–2121. - PubMed
-
- Conner DA. Mouse embryonic stem (ES) cell culture. Curr Protoc Mol Biol. 2001a;Chapter 23(Unit 23):23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

