Nutritional interventions for survivors of childhood cancer
- PMID: 27545902
- PMCID: PMC6486279
- DOI: 10.1002/14651858.CD009678.pub2
Nutritional interventions for survivors of childhood cancer
Abstract
Background: Childhood cancer survivors are at a higher risk of developing health conditions such as osteoporosis, and cardiovascular disease than their peers. Health-promoting behaviour, such as consuming a healthy diet, could lessen the impact of these chronic issues, yet the prevalence rate of health-protecting behaviour amongst survivors of childhood cancer is similar to that of the general population. Targeted nutritional interventions may prevent or reduce the incidence of these chronic diseases.
Objectives: The primary aim of this review was to assess the efficacy of a range of nutritional interventions designed to improve the nutritional intake of childhood cancer survivors, as compared to a control group of childhood cancer survivors who did not receive the intervention. Secondary objectives were to assess metabolic and cardiovascular risk factors, measures of weight and body fat distribution, behavioural change, changes in knowledge regarding disease risk and nutritional intake, participants' views of the intervention, measures of health status and quality of life, measures of harm associated with the process or outcomes of the intervention, and cost-effectiveness of the intervention
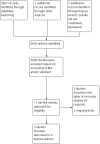
Search methods: We searched the electronic databases of the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 3), MEDLINE/PubMed (from 1945 to April 2013), and Embase/Ovid (from 1980 to April 2013). We ran the search again in August 2015; we have not yet fully assessed these results, but we have identified one ongoing trial. We conducted additional searching of ongoing trial registers - the International Standard Randomised Controlled Trial Number register and the National Institutes of Health register (both screened in the first half of 2013) - reference lists of relevant articles and reviews, and conference proceedings of the International Society for Paediatric Oncology and the International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer (both 2008 to 2012).
Selection criteria: We included all randomised controlled trials (RCTs) that compared the effects of a nutritional intervention with a control group which did not receive the intervention in this review. Participants were childhood cancer survivors of any age, diagnosed with any type of cancer when less than 18 years of age. Participating childhood cancer survivors had completed their treatment with curative intent prior to the intervention.
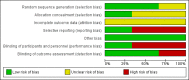
Data collection and analysis: Two review authors independently selected and extracted data from each identified study, using a standardised form. We assessed the validity of each identified study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. We used the GRADE criteria to assess the quality of each trial.
Main results: Three RCTs were eligible for review. A total of 616 participants were included in the analysis. One study included participants who had been treated for acute lymphoblastic leukaemia (ALL) (275 participants). Two studies included participants who had all forms of paediatric malignancies (266 and 75 participants). All participants were less than 21 years of age at study entry. The follow-up ranged from one month to 36 months from the initial assessment. All intended outcomes were not evaluated by each included study. All studies looked at different interventions, and so we were unable to pool results. We could not rule out the presence of bias in any of the studies.There was no clear evidence of a difference in calcium intake at one month between those who received the single, half-day, group-based education that focused on bone health, and those who received standard care (mean difference (MD) 111.60, 95% confidence interval (CI) -258.97 to 482.17; P = 0.56, low quality evidence). A regression analysis, adjusting for baseline calcium intake and changes in knowledge and self-efficacy, showed a significantly greater calcium intake for the intervention as compared with the control group at the one-month follow-up (beta coefficient 4.92, 95% CI 0.33 to 9.52; P = 0.04). There was statistically significant higher, self-reported milk consumption (MD 0.43, 95% CI 0.07 to 0.79; P = 0.02, low quality evidence), number of days on calcium supplementation (MD 11.42, 95% CI 7.11 to 15.73; P < 0.00001, low quality evidence), and use of any calcium supplementation (risk ratio (RR) 3.35, 95% CI 1.86 to 6.04; P < 0.0001, low quality evidence), with those who received this single, face-to-face, group-based, health behaviour session.There was no clear evidence of a difference in bone density Z-scores measured with a dual-energy X-ray absorptiometry (DEXA) scan at 36 months follow-up (MD -0.05, 95% CI -0.26 to 0.16; P = 0.64, moderate quality evidence) between those who received calcium and vitamin D supplementation combined with nutrition education and those who received nutrition education alone. There was also no clear evidence of a difference in bone mineral density between the intervention and the control group at the 12-month (median difference -0.17, P = 0.99) and 24-month follow-up (median difference -0.04, P = 0.54).A single multi-component health behaviour change intervention, focusing on general healthy eating principles, with two telephone follow-ups brought about a 0.17 lower score on the four-point Likert scale of self-reported junk food intake compared with the control group (MD -0.17, 95% CI -0.33 to -0.01; P = 0.04, low quality evidence); this result was statistically significant. There was no clear evidence of a difference between the groups in the self-reported use of nutrition as a health protective behaviour (MD -0.05, 95% CI -0.24 to 0.14; P = 0.60, low quality evidence).
Authors' conclusions: Due to a paucity of studies, and the heterogeneity of the studies included in this review, we are unable to draw conclusions regarding the effectiveness of nutritional interventions for use with childhood cancer survivors. Although there is low quality evidence for the improvement in health behaviours using health behaviour change interventions, there remains no evidence as to whether this translates into an improvement in dietary intake. There was also no evidence that the studies reduced the risk of cardiovascular and metabolic disorders in childhood cancer survivors, although no evidence of effect is not the same as evidence of no effect. This review highlights the need for further well designed trials to be implemented in this population.
Conflict of interest statement
Jennifer Cohen: None known.
Claire Wakefield: None Known.
Richard Cohn: None Known.
Figures





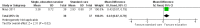
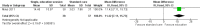







Update of
- doi: 10.1002/14651858.CD009678
Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Transition of care for adolescents from paediatric services to adult health services.Cochrane Database Syst Rev. 2016 Apr 29;4(4):CD009794. doi: 10.1002/14651858.CD009794.pub2. Cochrane Database Syst Rev. 2016. PMID: 27128768 Free PMC article.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Needs, Barriers and Facilitators of Adolescents Participating in a Lifestyle Promotion Program in Oncology: Stakeholders, Adolescents and Parents' Perspective.Children (Basel). 2022 Sep 1;9(9):1340. doi: 10.3390/children9091340. Children (Basel). 2022. PMID: 36138649 Free PMC article.
-
The Role of Nutrition in Primary and Secondary Prevention of Cardiovascular Damage in Childhood Cancer Survivors.Nutrients. 2022 Aug 11;14(16):3279. doi: 10.3390/nu14163279. Nutrients. 2022. PMID: 36014785 Free PMC article. Review.
-
Nutritional interventions in children with acute lymphoblastic leukemia undergoing antineoplastic treatment: a systematic review.BMC Nutr. 2024 Jun 19;10(1):89. doi: 10.1186/s40795-024-00892-4. BMC Nutr. 2024. PMID: 38898513 Free PMC article.
-
Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives.Cancers (Basel). 2022 Sep 6;14(18):4349. doi: 10.3390/cancers14184349. Cancers (Basel). 2022. PMID: 36139510 Free PMC article. Review.
-
L'évaluation et l'optimisation de la santé osseuse chez les enfants ayant des affections chroniques.Paediatr Child Health. 2022 Jul 18;27(4):232-242. doi: 10.1093/pch/pxac035. eCollection 2022 Jul. Paediatr Child Health. 2022. PMID: 35859683 Free PMC article.
References
References to studies included in this review
Cox 2005 {published data only}
-
- Cox CL, McLaughlin RA, Rai SN, Steen BD, Hudson MM. Adolescent survivors: A secondary analysis of a clinical trial targeting behavior change. Pediatric Blood and Cancer 2005;45:144‐54. - PubMed
Mays 2011 {published data only}
Rai 2008 {published data only}
-
- Hudson MM, Tyc VL, Jayawardene DA, Gattuso J, Quargnenti A, Greenwald C, et al. Feasibility of implementing health promotion interventions to improve health‐related quality of life. International Journal of Cancer 1999;Suppl 12:138‐42. - PubMed
-
- Hudson MM, Tyc VL, Srivastava DK, Gattuso J, Quargnenti A, Crom DB, et al. Multi‐component behavioral intervention to promote health protective behaviors in childhood cancer survivors: The protect study. Medical and Pediatric Oncology 2002;39:2‐11. - PubMed
-
- Kaste SC, Qi A, Surprise H, Lovorn E, Boyett J, Ferry Jr RJ, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatric Blood and Cancer 2014;61:885‐93. - PMC - PubMed
-
- Rai SN, Hudson MM, McCammon E, Carbone L, Tylavsky F, Smith K, et al. Implementing an intervention to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia: BONEII, a prospective placebo‐controlled double‐blind randomized interventional longitudinal study design. Contemporary Clinical Trials 2008;29:711‐9. - PMC - PubMed
References to studies excluded from this review
Mays 2012 {published data only}
Moyer‐Mileur 2009 {published data only}
-
- Moyer‐Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard‐risk acute lymphoblastic leukemia during maintenance therapy. Journal of Pediatric Hematology Oncology 2009;31:259‐66. - PubMed
References to ongoing studies
NCT01473342 {published data only}
-
- NCT01473342. Mila Blooms intervention study: an app for promoting physical activity and health diet among adolescent survivors of childhood cancer. https://clinicaltrials.gov/ct2/show/NCT01473342 (accessed 2013). - PMC - PubMed
Additional references
Arroyave 2008
-
- Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviours. Oncology Nursing Forum 2008;35(1):121‐30. - PubMed
Braam 2013a
-
- Braam KI, Torre P, Takken T, Veening MA, Dulmen‐den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008796.pub2; CD008796] - DOI - PubMed
Brunner 2009
Cohen 2012
-
- Cohen J, Wakefield CE, Fleming CA, Gawthorne R, Tapsell LC, Cohn RJ. Dietary intake after treatment in child cancer survivors. Pediatric Blood and Cancer 2012;58(5):752‐5. - PubMed
Cox 2009
Demark‐Wahnefried 2005
-
- Demark‐Wahnefried W, Werner C, Clipp EC, Guill AB, Bonner M, Jones LW, et al. Survivors of childhood cancer and their guardians. Cancer 2005;103(10):2171‐80. - PubMed
Dickerman 2007
-
- Dickerman JD. The late effects of childhood cancer therapy. Pediatrics 2007;119(3):554‐68. - PubMed
Diller 2009
Emond 2010
-
- Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics 2010;126(2):e337‐42. - PubMed
Friedman 2006
-
- Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatric Blood and Cancer 2006;46:159‐68. - PubMed
Guyatt 2008a
Guyatt 2008b
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
Hudson 2002
-
- Hudson MM, Tyc VL, Srivastava DK, Gattuso J, Quargnenti A, Crom DB, et al. Multi‐component behavioral intervention to promote health protective behaviors in childhood cancer survivors: The Protect Study. Pediatric Blood & Cancer 2002;39(1):2‐11. - PubMed
Hudson 2009
Jemal 2009
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M J. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians 2009;59(4):225‐49. - PubMed
Jones 2010
Kuhn 2004
-
- Kuhn DE, Matson JL. Assessment of feeding and mealtime behavior problems in persons with mental retardation. Behavior Modification 2004;28(5):638‐48. - PubMed
Lakka 2007
-
- Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Applied Physiology, Nutrition, and Metabolism 2007;32(1):76‐88. - PubMed
Landier 2006
-
- Landier W, Wallace WHB, Hudson MM. Long‐term follow‐up of pediatric cancer survivors: education, surveillance, and screening. Pediatric Blood and Cancer 2006;46:149‐58. - PubMed
Lewis 2004
-
- Lewis E, Kritzinger A. Parental experiences of feeding problems in their infants with Down syndrome. Down Dyndrome Research and Practice 2004;9(2):45‐52. - PubMed
Module CCG 2014
-
- Kremer LCM, Leclercq E, Dalen EC. Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2014, Issue 11. Art. No.: CHILDCA.
Mulhern 1995
-
- Mulhern RK, Tyc VL, Phipps S, Crom DB, Barclay D, Greenwald C, et al. Health‐related behaviours of survivors of childhood cancer. Medical and Pediatric Oncology 1995;27(14):159‐65. - PubMed
Mulrooney 2009
Ness 2007
-
- Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annual Review of Public Health 2007;28:279‐302. - PubMed
Oeffinger 2006
-
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine 2006;355(15):1572‐82. - PubMed
Oeffinger 2008
-
- Oeffinger KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased risk of cardiovascular disease?. Pediatric Blood and Cancer 2008;50(Suppl 2):462‐7. - PubMed
Oeffinger 2009
-
- Oeffinger KC, Hudson MM, Landier W. Survivorship: childhood cancer survivors. Primary Care 2009;36(4):743‐80. - PubMed
Oude Luttikhuis 2009
Pereira 2009
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robien 2008
Siviero‐Miachon 2008
Skinner 2006
-
- Skinner R, Wallace WHB, Levitt GA. Long‐term follow‐up of people who have survived cancer during childhood. Lancet Oncology 2006;7(6):489‐98. - PubMed
Steinberger 2012
Stolley 2009
Stolley 2010
Tota‐Maharaj 2010
-
- Tota‐Maharaj R, Defilippis AP, Blumenthal RS, Blaha MJ. A practical approach to the metabolic syndrome: review of current concepts and management. Current Opinion in Cardiology 2010;25:502‐12. - PubMed
Zelen 1974
-
- Zelen M. The randomization and stratification of patients to clinical trials. Journal of Chronic Disease 1974;27:365‐75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

