Steroid avoidance or withdrawal for kidney transplant recipients
- PMID: 27546100
- PMCID: PMC8520739
- DOI: 10.1002/14651858.CD005632.pub3
Steroid avoidance or withdrawal for kidney transplant recipients
Abstract
Background: Steroid-sparing strategies have been attempted in recent decades to avoid morbidity from long-term steroid intake among kidney transplant recipients. Previous systematic reviews of steroid withdrawal after kidney transplantation have shown a significant increase in acute rejection. There are various protocols to withdraw steroids after kidney transplantation and their possible benefits or harms are subject to systematic review. This is an update of a review first published in 2009.
Objectives: To evaluate the benefits and harms of steroid withdrawal or avoidance for kidney transplant recipients.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 15 February 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria: All randomised and quasi-randomised controlled trials (RCTs) in which steroids were avoided or withdrawn at any time point after kidney transplantation were included.
Data collection and analysis: Assessment of risk of bias and data extraction was performed by two authors independently and disagreement resolved by discussion. Statistical analyses were performed using the random-effects model and dichotomous outcomes were reported as relative risk (RR) and continuous outcomes as mean difference (MD) with 95% confidence intervals.
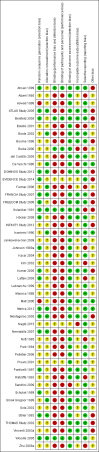
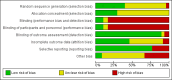
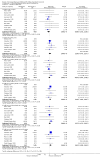
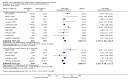
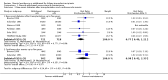
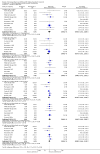
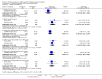
Main results: We included 48 studies (224 reports) that involved 7803 randomised participants. Of these, three studies were conducted in children (346 participants). The 2009 review included 30 studies (94 reports, 5949 participants). Risk of bias was assessed as low for sequence generation in 19 studies and allocation concealment in 14 studies. Incomplete outcome data were adequately addressed in 22 studies and 37 were free of selective reporting.The 48 included studies evaluated three different comparisons: steroid avoidance or withdrawal compared with steroid maintenance, and steroid avoidance compared with steroid withdrawal. For the adult studies there was no significant difference in patient mortality either in studies comparing steroid withdrawal versus steroid maintenance (10 studies, 1913 participants, death at one year post transplantation: RR 0.68, 95% CI 0.36 to 1.30) or in studies comparing steroid avoidance versus steroid maintenance (10 studies, 1462 participants, death at one year after transplantation: RR 0.96, 95% CI 0.52 to 1.80). Similarly no significant difference in graft loss was found comparing steroid withdrawal versus steroid maintenance (8 studies, 1817 participants, graft loss excluding death with functioning graft at one year after transplantation: RR 1.17, 95% CI 0.72 to 1.92) and comparing steroid avoidance versus steroid maintenance (7 studies, 1211 participants, graft loss excluding death with functioning graft at one year after transplantation: RR 1.09, 95% CI 0.64 to 1.86). The risk of acute rejection significantly increased in patients treated with steroids for less than 14 days after transplantation (7 studies, 835 participants: RR 1.58, 95% CI 1.08 to 2.30) and in patients who were withdrawn from steroids at a later time point after transplantation (10 studies, 1913 participants, RR 1.77, 95% CI 1.20 to 2.61). There was no evidence to suggest a difference in harmful events, such as infection and malignancy, in adult kidney transplant recipients. The effect of steroid withdrawal in children is unclear.
Authors' conclusions: This updated review increases the evidence that steroid avoidance and withdrawal after kidney transplantation significantly increase the risk of acute rejection. There was no evidence to suggest a difference in patient mortality or graft loss up to five year after transplantation, but long-term consequences of steroid avoidance and withdrawal remain unclear until today, because prospective long-term studies have not been conducted.
Conflict of interest statement
MCH: none known
AR: none known
EVN: none known
JP: none known
ACW: none known
Figures














Update of
-
Steroid avoidance or withdrawal for kidney transplant recipients.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD005632. doi: 10.1002/14651858.CD005632.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2016 Aug 22;(8):CD005632. doi: 10.1002/14651858.CD005632.pub3. PMID: 19160257 Updated.
Comment in
-
Avoiding or stopping steroids in kidney transplant recipients: sounds good but does it work?Cochrane Database Syst Rev. 2016 Aug 30;8(8):ED000114. doi: 10.1002/14651858.ED000114. Cochrane Database Syst Rev. 2016. PMID: 27595295 Free PMC article. No abstract available.
References
References to studies included in this review
Ahsan 1999 {published data only}
-
- Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil ‐ a prospective randomized study. Steroid Withdrawal Study Group. Transplantation 1999;68(12):1865‐74. [MEDLINE: ] - PubMed
-
- Matas A, Ewell M, Cooperative Clinical Trials in Adult Transplantation Group. Prednisone withdrawal in kidney transplant recipients on CSA/MMF ‐ a prospective randomized study [abstract no: 4]. Transplantation 1999;67(9):S543. [CENTRAL: CN‐00765998] - PubMed
Albert 1985 {published data only}
-
- Albert FW, Schmidt U. Cyclosporin A (Cy A) therapy with or without steroids in cadaveric kidney transplantation. A prospective randomized one‐center study [abstract]. Kidney International 1985;28(4):708. [CENTRAL: CN‐00550573]
-
- Albert FW, Schmidt U. Cyclosporine therapy with or without steroids in cadaveric kidney transplantation ‐ A prospective randomized one‐center study. Transplantation Proceedings 1985;17(6):2669‐70. [EMBASE: 1986055112]
Aswad 1998 {published data only}
-
- Aswad S, Zapanta R, Wu L, Bogaard T, Asai P, Khetan U, et al. Steroid withdrawal in living related kidney transplant patients receiving FK506 [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:394. [CENTRAL: CN‐00483058]
ATLAS Study 2005 {published data only}
-
- Klinger M, Vitko S, Salmela K, Wlodarczyk Z, Tyden G, ATLAS Study Group. Large, prospective study evaluating steroid‐free immunosuppression with tacrolimus/basiliximab and tacrolimus/MMF compared with tacrolimus/MMF/steroids in renal transplantation [abstract no: W748]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788‐9. [CENTRAL: CN‐00446121]
-
- Kramer BK, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Vitko S. Two steroid‐free immunosuppressive regimens (basiliximab/tacrolimus and tacrolimus/MMF) in comparison to tacrolimus/MMF/steroid therapy after renal transplantation [abstract no: F‐FC039]. Journal of the American Society of Nephrology 2003;14(Nov):9A. [CENTRAL: CN‐00583329]
-
- Kramer BK, Klinger M, Vitko S, Glyda M, Midtvedt K, Stefoni S, et al. Tacrolimus‐based, steroid‐free regimens in renal transplantation: 3‐year follow‐up of the ATLAS trial. Transplantation 2012;94(5):492‐8. [MEDLINE: ] - PubMed
-
- Kramer BK, Klinger M, Wlodarczyk Z, Ostrowski M, Midvedt K, Stefoni S, et al. Tacrolimus combined with two different corticosteroid‐free regimens compared with a standard triple regimen in renal transplantation: one year observational results. Clinical Transplantation 2010;24(1):E1‐9. [MEDLINE: ] - PubMed
-
- Kramer BK, Kruger B, Hoffmann U, Wlodarcyzk Z, Tyden G, Senatorski G, et al. 1‐year‐follow‐up of two steroid‐free immunosuppressive regimens ‐ basiliximab/tacrolimus and tacrolimus/MMF ‐ in comparison to tacrolimus/MMF/steroids after renal transplantation [abstract no: F‐PO1026]. Journal of the American Society of Nephrology 2004;15(Oct):289A. [CENTRAL: CN‐00688822]
Benfield 2005 {published data only}
-
- Benfield MR, Bartosh S, Ikle D, Warshaw B, Bridges N, Morrison Y, et al. A randomized double‐blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation. American Journal of Transplantation 2010;10(1):81‐8. [MEDLINE: ] - PubMed
-
- Benfield MR, Munoz R, Warshaw BL, Bartosh SM, Stablein DM, McIntosh MJ, et al. A randomized controlled double‐blind trial of steroid withdrawal in pediatric renal transplantation: a study of the Cooperative Clinical trials in pediatric transplantation (CCTPT) [abstract no: 966]. American Journal of Transplantation 2005;5(Suppl 11):402. [CENTRAL: CN‐00676069]
-
- McDonald RA, McIntosh M, Stablein D, Grimm P, Wyatt R, Lirenman D, et al. Increased incidence of PTLD in pediatric renal transplant recipients enrolled in a randomized controlled trial of steroid withdrawal: a study of the CCTPT [abstract no: 1028]. American Journal of Transplantation 2005;5(Suppl 11):418.
-
- McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. American Journal of Transplantation 2008;8(5):984‐9. [MEDLINE: ] - PubMed
Boletis 2001 {published data only}
-
- Boletis JN, Konstadinidou I, Chelioti H, Theodoropoulou H, Avdikou K, Kostakis A, et al. Successful withdrawal of steroid after renal transplantation. Transplantation Proceedings 2001;33(1‐2):1231‐3. [MEDLINE: ] - PubMed
-
- Boletis JN, Konstadinidou I, Chelioti H, Theodoropoulou H, Avdikou K, Kostakis A, et al. Successful withdrawal of steroids after renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00444474]
-
- Boletis JN, Konstadinidou I, Darema M, Psimenou E, Chiras T, Kostakis A, et al. Steroids withdrawal in renal transplant recipients: a randomized controlled study [abstract no: T447]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):312. [CENTRAL: CN‐00509095]
Boots 2002 {published data only}
-
- Boots JM, Christaiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplant recipients with tacrolimus dual therapy [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415308]
-
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation 2002;74(12):1703‐9. [MEDLINE: ] - PubMed
-
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen R, Hooff JP. Early steroid withdrawal in renal transplant recipients with tacrolimus double therapy immunosuppression [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):878A. [CENTRAL: CN‐00550471]
-
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation Proceedings 2002;34(5):1698‐9. [MEDLINE: ] - PubMed
Bouma 1996 {published data only}
-
- Bouma GJ, Hollander DA, Meer‐Prins EM, Bree SP, Rood JJ, Woude FJ, et al. In vitro sensitivity to prednisolone may predict kidney rejection after steroid withdrawal. Transplantation 1996;62(10):1422‐9. [MEDLINE: ] - PubMed
-
- Bouma GJ, Hollander DJ, Doxiadis IN, Meer‐Prins EM, Bree SP, Rood JJ, et al. In vitro study: prediction of graft rejection after withdrawal of steroids [In vitro‐untersuchungen zur voraussage von transplantatabstossungen nach steroid‐sbsetzung: eine pilot‐studie]. Transplantationsmedizin: Organ Der Deutschen Transplantationsgesellschaft 1996;8(2):79‐83. [EMBASE: 1996240201]
-
- Hollander AA, Hene RJ, Hermans J, Es LA, Woude FJ. Late prednisone withdrawal in cyclosporine‐treated kidney transplant patients: a randomized study. Journal of the American Society of Nephrology 1997;8(2):294‐301. [MEDLINE: ] - PubMed
-
- Hollander AA, Hene RJ, Es LA, Woude FJ. Late prednisone withdrawal in renal transplant patients [abstract no: A3323]. Journal of the American Society of Nephrology 1996;7(9):1911. - PubMed
-
- Hollander AA, Hene RJ, Es LA, Woude FJ. Late prednisone withdrawal in renal transplant patients [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A276. [CENTRAL: CN‐00261334]
Burke 2000 {published data only}
-
- Burke J, Francos BB, Francos GC. Double‐blind, placebo‐controlled trial of steroid withdrawal in kidney transplant recipients with a cyclosporine/mycophenolate regimen‐three year follow up [abstract]. American Journal of Transplantation 2001;1(Suppl 1):296. [CENTRAL: CN‐00764555]
-
- Burke JF, Francos GC, Francos BB, Michael B, Gaughan WJ. A double‐blind, placebo‐controlled, three‐year study of steroid withdrawal using a neoral‐based immunosuppressive regimen in primary renal transplant recipients: an interim report [abstract no: 426]. Transplantation 2000;69(8 Suppl):S224. [CENTRAL: CN‐00444589]
-
- Francos GC, Frankel CJ, Dunn SR, Francos BB, Burke JF. Double‐blind, placebo‐controlled, 3 year study of steroid withdrawal using a neoral and mycophenolate mofetil (MMF)‐based immunosuppressive regimen in primary renal transplant recipients [abstract no: 137]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00415671]
del Castillo 2005 {published data only}
-
- Castillo D, Franco A, Tabernero JM, Errasti P, Valdes F, García C, et al. Prospective, multicenter, randomized, open‐label study of myfortic with steroid withdrawal vs myfortic with standard steroid regimen to prevent acute rejection in de novo kidney transplantation [abstract no: 136]. American Journal of Transplantation 2005;5(Suppl 11):191. [CENTRAL: CN‐00644130]
De Vecchi 1986 {published data only}
-
- Vecchi A, Tarantino A, Montagnino G, Aroldi A, Aniasi A, Vegeto A, et al. Ciclosporin alone or combined with steroid in the treatment of cadaveric renal transplant recipients. Clinical Transplantation 1987;1:198‐202. [CENTRAL: CN‐00764108]
-
- Vecchi A, Tarantino A, Rivolta E, Egidi F, Montagnino G, Berardinelli L, et al. Ciclosporin alone or associated with steroid for immunosuppression of cadaveric renal transplants?. Contributions to Nephrology 1986;51:88‐90. [MEDLINE: ] - PubMed
-
- Vecchi A, Tarantino A, Rivoltan E, Egidi F, Ponticelli C. Need for steroid in cyclosporine (Cy) treated cadaveric renal transplant recipients (pts) [abstract]. Kidney International 1985;28(2):394. [CENTRAL: CN‐00550392]
DOMINOS Study 2012 {published data only}
-
- Thierry A, Mourad G, Buchler M, Kamar N, Villemain F, Heng AE, et al. Steroid avoidance with early intensified dosing of enteric‐coated mycophenolate sodium: a randomized multicentre trial in kidney transplant recipients. Nephrology Dialysis Transplantation 2012;27(9):3651‐9. [MEDLINE: ] - PubMed
-
- Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F. Multicenter, randomized, comparative, open‐label study to evaluate efficacy and safety a combination of anti‐IL2R, intensified dose of enteric‐coated mycophenolate sodium (EC‐MPS) for 6 weeks, ciclosporine micro‐emulsion (CsA‐ME), with or without steroids, in adult kidney de novo transplant recipients (TxR) [abstract no: 1679]. American Journal of Transplantation 2010;10(Suppl 4):515.
-
- Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F, Heng AE, et al. Intensified dose of enteric‐coated mycophenolate sodium (EC‐MPS) for steroids avoidance, in combination with ciclosporine micro‐emulsion (CsA‐ME): multicenter, randomized, open label, comparative study in de novo kidney transplantation (DOMINOS) [abstract no: P‐557]. Transplant International 2009;22(Suppl 2):232‐3.
EVIDENCE Study 2014 {published data only}
-
- Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve‐month outcomes from EVIDENCE trial (everolimus once‐a‐day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients [abstract no: O193]. Transplant International 2013;26(Suppl 2):100. [EMBASE: 71359334]
-
- Ponticelli C, Carmellini M, Tisone G, Sandrini S, Segoloni G, Rigotti P, et al. A randomized trial of everolimus and low‐dose cyclosporine in renal transplantation: with or without steroids?. Transplantation Proceedings 2014;46(10):3375‐82. [MEDLINE: ] - PubMed
Farmer 2006 {published data only}
-
- Farmer C, Abbs I, Hilton R, et al. What is the value of short synacthen tests in predicting the ease of steroid withdrawal in renal transplant recipients? A randomised controlled trial [abstract]. American Journal of Transplantation 2001;1(Suppl 1):190. [CENTRAL: CN‐00767027]
-
- Farmer CK, Hampson G, Abbs IC, Hilton RM, Koffman CG, Fogelman I, et al. Late low‐dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. American Journal of Transplantation 2006;6(12):2929‐36. [MEDLINE: ] - PubMed
FRANCIA Study 2007 {published data only}
-
- Albano L, Cantarovich D, Rostaing L, Kamar N, Ducloux D, Mourad G, et al. Corticosteroid avoidance in adult kidney transplant recipients receiving ATG Fresenius induction: 5 years results of a prospective and randomized study [abstract no: P497]. Transplant International 2013;26(Suppl 2):285. [EMBASE: 71360109]
-
- Cantarovich D. Steroid avoidance in adult kidney transplant recipients: 5‐year results of a prospective and randomized multicenter study [abstract no: 234]. American Journal of Transplantation 2013;13(Suppl S5):101‐2. [EMBASE: 71056810]
-
- Cantarovich D, FRANCIA French Study Group. ATG‐Fresenius induction and immunosuppression without steroids following renal transplantation: a prospective and randomized study [abstract no: P189]. Transplant International 2007;20(Suppl 2):141. [CENTRAL: CN‐00740578]
-
- Cantarovich D, FRANCIA Study Group. Acute renal rejections are increased in the absence of corticosteroids but are not detrimental at one year in the context of ATG induction [abstract no: 515]. American Journal of Transplantation 2010;10(Suppl 4):191. [EMBASE: 70463876]
-
- Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint‐Hillier Y, Mourad G, et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG‐F induction: 5‐year actual results of a prospective and randomized study. American Journal of Transplantation 2014;14(11):2556‐64. [MEDLINE: ] - PubMed
FREEDOM Study 2008 {published data only}
-
- Chadban S, Walker R, Russ G, Kanellis J. Renal functions and rejection incidence in de novo renal transplant patients randomised to steroid avoidance, steroid withdrawal or standard steroids [abstract]. Immunology & Cell Biology 2007;85(4):A24.
-
- Schena FP, Vincenti F, Paraskevas S, Hauser I, FREEDOM Study Group. Renal function and rejection incidence in de novo renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids [abstract no: F‐FC153]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601969]
-
- Schena FP, Vincenti F, Paraskevas S, Hauser I, Grinyo J, FREEDOM Study Group. 12‐month results of a prospective, randomized trial of steroid avoidance, steroid withdrawal or standard steroids in de novo renal transplant patients receiving cyclosporine, enteric‐coated mycophenolate sodium (EC‐MPS, myfortic®) and basiliximab [abstract no: 54]. American Journal of Transplantation 2006;6(Suppl 2):84‐5. [CENTRAL: CN‐00644263]
-
- Vincenti F, Schena F, Walker R, Pescovitz M, Shoker A. 3 months interim results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS, Myfortic®), basiliximab, and neoral C‐2 comparing different steroid protocols in de novo kidney recipients [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2005;16(Oct):236A. [CENTRAL: CN‐00583476]
-
- Vincenti F, Schena FP, Paraskevas S, Hauser I, FREEDOM Study Group. Comparison of metabolic parameters in renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids with a 12‐month, randomized, multicenter trial [abstract no: F‐PO1076]. Journal of the American Society of Nephrology 2006;17(Abstracts):562A. [CENTRAL: CN‐00602097]
Gulanikar 1991 {published data only}
-
- Gulanikar AC, Belitsky P, MacDonald AS, Cohen A, Bitter‐Suermann H. Randomized controlled trial of steroids versus no steroids in stable cyclosporine‐treated renal graft recipients. Transplantation Proceedings 1991;23(1 Pt 2):990‐1. [MEDLINE: ] - PubMed
Höcker 2009 {published data only}
-
- Hoecker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective randomized trial on late steroid withdrawal in pediatric renal transplant recipients under CsA and MMF: 2‐year data [abstract no: 605]. American Journal of Transplantation 2009;9(Suppl 2):366. [EMBASE: 70010478] - PubMed
-
- Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2‐year results of a prospective, randomised trial in paediatric renal transplantation. Nephrology Dialysis Transplantation 2010;25(2):617‐24. [MEDLINE: ] - PubMed
-
- Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation 2009;87(6):934‐41. [MEDLINE: ] - PubMed
-
- Tönshoff B, Weber L, Höcker B. Prospective randomized multicenter trial on withdrawal of steroids in pediatric renal transplant recipients with stable graft function on cyclosporin A (CsA) and mycophenolate mofetil (MMF) [abstract no: 240 (SY)]. Pediatric Nephrology 2007;22(9):1429.
-
- Weber LT, Hoecker B, Feneberg R, Drube J, John U, Fehrenbach H, et al. Late steroid withdrawal in pediatric renal transplant recipients under cyclosporin microemulsion and mycophenolate mofetil [abstract no: SA‐PO2535]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):679A.
INFINITY Study 2013 {published data only}
-
- Thierry A, Mourad G, Buchler M, Kamar N, Villemain F, Heng A, et al. Three‐year safety and efficacy outcomes in kidney transplant patients randomized to steroid avoidance or maintenance steroids with early intensified dosing of enteric‐coated mycophenolate sodium: The INFINITY Study [abstract no: B1099]. American Journal of Transplantation 2013;13(Suppl S5):358. [EMBASE: 71057675]
Isoniemi 1990 {published data only}
-
- Isoniemi H. Renal allograft immunosuppression V: Glucose intolerance occurring in different immunosuppressive treatments. Clinical Transplantation 1991;5(3):268‐72. [EMBASE: 1991187379]
-
- Isoniemi H. Renal allograft immunosuppression. III. Triple therapy versus three different combinations of double drug treatment: two year results in kidney transplant patients. Transplant International 1991;4(1):31‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Ahonen J, Eklund B, Hockerstedt K, Salmela K, Willebrand E, et al. Renal allograft immunosuppression. II. A randomized trial of withdrawal of one drug in triple drug immunosuppression. Transplant International 1990;3(3):121‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Ahonen J, Krogerus L, Eklund B, Hockerstedt K, Salmela K, et al. Chronic rejection of renal allografts with four immunosuppressive regimens. Transplantation Proceedings 1992;24(6):2716‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Eklund B, Hockerstedt K, Korsback C, Salmela K, Willebrand E, et al. Discontinuation of one drug in triple drug treatment of renal allograft patients: 1‐year results. Transplantation Proceedings 1990;22(4):1365‐6. [MEDLINE: ] - PubMed
Jankowska‐Gan 2009 {published data only}
Johnson 1989a {published data only}
-
- Johnson RW, Mallick NP, Bakran A, Pearson RC, Scott PD, Dyer P, et al. Cadaver renal transplantation without maintenance steroids. Transplantation Proceedings 1989;21(1 Pt 2):1581‐2. [MEDLINE: ] - PubMed
-
- Johnson RW, Mallick NP, Scott PD, Riad H, Pearson RC, Dyer P, et al. Prospective trials with cyclosporine monotherapy in cadaver renal transplantation. Journal of Nephrology 1990;3(4 Suppl 1):47‐9. [EMBASE: 1992097616]
Kacar 2004 {published data only}
-
- Kacar S, Gurkan A, Karaoglan M, Akman F, Varilsuha C, Karaca C, et al. Steroid withdrawal protocol in renal transplantation [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:401. [CENTRAL: CN‐00509258]
Kim 2002 {published data only}
-
- Kim EH, Gohh R, Morrissey P, Simpson M, Monaco A, Yango A. Rapid steroid withdrawal versus standard steroid treatment in patients treated with basiliximab, cyclosporine, and mycophenolate mofetil for the prevention of acute rejection in kidney transplantation: a 2‐year follow‐up [abstract no: 1029]. American Journal of Transplantation 2002;2(Suppl 3):397. [CENTRAL: CN‐00416017]
Kumar 2005 {published data only}
-
- Fa K, Kode RK, Lu Q, Kumar MS, Laftavi MR, Pankewycz OG. Value of one month protocol biopsies combined with a molecular analysis in predicting efficacy of rapid steroid withdrawal after renal transplantation [abstract no: 132]. American Journal of Transplantation 2002;2(Suppl 3):171. [CENTRAL: CN‐00415620]
-
- Fa K, Laftavi MR, Ferry E, Kumar AM, Fyfe B, Pankewycz OG. The predictive value of subclinical rejection in a steroid free immunosuppressive regimen [abstract no: 1282]. American Journal of Transplantation 2003;3(Suppl 5):480. [CENTRAL: CN‐00445267]
-
- Kumar MS, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):393.
-
- Kumar MS, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF)‐a randomized prospective controlled clinical trial [abstract no: 2440]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416079]
-
- Kumar MS, Heifets M, Moritz MJ, Parikh MH, Saeed MI, Lingaraju R, et al. Basiliximab induction in African American recipients (AA) of cadaver kidneys (CAD) facilitates steroid avoidance, reduces acute rejection (AR) and prolongs survival [abstract no: 1627]. American Journal of Transplantation 2005;5(Suppl 11):570. [CENTRAL: CN‐00644172]
Laftavi 2005 {published data only}
-
- Laftavi M, Stefanick B, Stephan R, Kohli R, Min I, Sridhar N, et al. The significance of protocol biopsy in immunominimization protocol: a prospective study of steroid withdrawal [abstract no: O229]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509305]
-
- Laftavi MR, Leca N, Dagher F, Kilmer L, Stephanick B, Kohli R, et al. Steroid withdrawal is associated with more chronic allograft nephropathy (CAN) following kidney transplantation [abstract no: 518]. American Journal of Transplantation 2005;5(Suppl 11):288.
-
- Laftavi MR, Stephan R, Stefanick B, Kohli R, Dagher F, Applegate M, et al. Randomized prospective trial of early steroid withdrawal compared with low‐dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 2005;137(3):364‐71. [MEDLINE: ] - PubMed
-
- Pankewycz OG, Stephan R, Stefanick B, Dagher F, Applegate M, Kohli R, et al. The clinical benefits of early steroid withdrawal (7 days) and utility of protocol biopsies at 1, 6 and 12 months in guiding steroid‐free immunosuppressive therapy after renal transplantation [abstract no: 1534]. American Journal of Transplantation 2004;4(Suppl 8):579. [CENTRAL: CN‐00509401]
-
- Pankewyez O, Stephan R, Stefanick B, Rubino A, Kohli R, Min I, et al. Induction immunosuppression for renal transplantation using thymoglobulin, FK506 and mycophenolate mofetil allows for safe steroid withdrawal and eliminates the need for early protocol biopsies [abstract no: 1271]. American Journal of Transplantation 2003;3(Suppl 5):478. [CENTRAL: CN‐00447092]
Lebranchu 1999 {published data only}
-
- Hene RJ, M55002 Study Group. A randomized, double‐blind, multi‐center trial comparing two corticosteroid regimens in combination with mycophenolate mofetil (MMF) and cyslosporine (CYA) in renal transplant recipients [abstract no: 420]. Transplantation 1998;65(12):S107. [CENTRAL: CN‐00763818]
-
- Lebranchu Y. Comparison of two corticosteroid regimens in combination with CellCept and cyclosporine A for prevention of acute allograft rejection: 12 month results of a double‐blind, randomized, multi‐center study. M 55002 Study Group. Transplantation Proceedings 1999;31(1‐2):249‐50. [MEDLINE: ] - PubMed
-
- Lebranchu Y, Aubert P, Bayle F, Bedrossian J, Berthoux F, Bourbigot B, et al. Could steroids be withdrawn in renal transplant patients sequentially treated with ATG, cyclosporine, and cellcept? One‐year results of a double‐blind, randomized, multicenter study comparing normal dose versus low‐dose and withdrawal of steroids. M 55002 French Study Group. Transplantation Proceedings 2000;32(2):396‐7. [MEDLINE: ] - PubMed
-
- Lebranchu Y, M55002 Study Group. A comparison of two corticosteroid regimens in kidney transplanted patients treated with ATG/OKT3 induction, mycophenolate mofetil (MMF) and cyclosporine A (CyA) for prevention of acute allograft rejection. 12 months results of a double‐blind, randomized, multi‐center study [abstract no: 932]. Transplantation 1999;67(7):S239. [CENTRAL: CN‐00763351] - PubMed
-
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Kukula K, Lewandowska D, Baczkowska T, et al. Elevated serum immunoglobulins after steroid withdrawal in renal allograft recipients. Transplantation Proceedings 2002;34(2):564‐6. [MEDLINE: ] - PubMed
Maiorca 1988 {published data only}
-
- Cristinelli L, Brunori G, Manganoni AM, Manganoni A, Setti G, Maiorca R. Controlled study of steroid withdrawal after 6 months in renal transplant patients treated with ciclosporin. Contributions to Nephrology 1986;51:91‐5. [MEDLINE: ] - PubMed
-
- Cristinelli L, Brunori G, Setti G, Manganoni A, Manganoni AM, Scolari F, et al. Withdrawal of methylprednisolone at the sixth month in renal transplant recipients treated with cyclosporine. Transplantation Proceedings 1987;19(1 Pt 3):2021‐3. [MEDLINE: ] - PubMed
-
- Cristinelli L, Brunori G, Setti G, Scolari F, Scaini P, Manganoni S, et al. Controlled randomised trial of methylprednisolone withdrawal at the sixth month in renal transplant recipients treated with cyclosporin [abstract]. Nephrology Dialysis Transplantation 1986;1(2):139. [CENTRAL: CN‐00260301]
-
- Maiorca R, Cristinelli L, Brunori G, Setti G, Salerni B, Nobili U, et al. Prospective controlled trial of steroid withdrawal after six months in renal transplant patients treated with cyclosporine. Transplantation Proceedings 1988;20(3 Suppl 3):121‐5. [MEDLINE: ] - PubMed
Matl 2000 {published data only}
-
- Matl I, Lacha J, Lodererova A, Simova M, Teplan V, Lanska V, et al. Withdrawal of prednisone from a triple combination of immunosuppressive agents after kidney transplantation [Vysazení prednisonu z trojkombinace imunosupresiv u nemocných po transplantaci ledviny]. Casopis Lekaru Ceskych 2000;139(4):115‐9. [MEDLINE: ] - PubMed
-
- Matl I, Lacha J, Lodererova A, Simova M, Teplan V, Lanska V, et al. Withdrawal of steroids from triple‐drug therapy in kidney transplant patients. Nephrology Dialysis Transplantation 2000;15(7):1041‐5. [MEDLINE: ] - PubMed
-
- Matl I, Lacha J, Lodererova A, Simova M, Vitko S. Withdrawal of steroids from triple drug therapy in renal transplant patients [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:351. [CENTRAL: CN‐00485004] - PubMed
Mericq 2013 {published data only}
-
- Mericq V, Salas P, Pinto V, Cano F, Reyes L, Brown K, et al. Steroid withdrawal in pediatric kidney transplant allows better growth, lipids and body composition: a randomized controlled trial. Hormone Research in Pædiatrics 2013;79(2):88‐96. [MEDLINE: ] - PubMed
Montagnino 2005 {published data only}
-
- Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplantation Proceedings 2005;37(2):788‐90. [MEDLINE: ] - PubMed
-
- Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, et al. A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low‐dose cyclosporine. Nephrology Dialysis Transplantation 2008;23(2):707‐14. [MEDLINE: ] - PubMed
-
- Ponticelli C, Sandrini S, Casciani C, Schena FP, STAR Group. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. [abstract no: O432]. Transplantation 2004;78(2 Suppl):170. [CENTRAL: CN‐00509422] - PubMed
Nagib 2015 {published data only}
-
- Nagib AM, Abbas MH, Abu‐Elmagd MM, Denewar AA, Neamatalla AH, Refaie AF, et al. Long‐term study of steroid avoidance in renal transplant patients: a single‐center experience. Transplant Proceedings 2015;47(4):1099‐104. [MEDLINE: ] - PubMed
-
- Nagib AM, Neamatalla AH, Bakr MA, Refaie AF, Abo‐Elmagd MM, Wafa IW. Long term study of steroid avoidance in renal transplant patients: A single center experience [abstract]. Experimental & Clinical Transplantation 2014;12:100. [EMBASE: 71976229] - PubMed
Nematalla 2007 {published data only}
-
- Gheith OA, Nematalla AH, Bakr MA, Refaie A, Shokeir AA, Ghoneim MA. Cost‐benefit of steroid avoidance in renal transplant patients: a prospective randomized study. Scandinavian Journal of Urology & Nephrology 2010;44(3):175‐82. [MEDLINE: ] - PubMed
-
- Gheith OA, Nematalla AH, Bakr MA, Refaie A, Shokeir AA, Ghoneim MA. Steroid avoidance reduce the cost of morbidities after live‐donor renal allotransplants: a prospective, randomized, controlled study. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2011;9(2):121‐7. [MEDLINE: ] - PubMed
-
- Neamatalla A, Bakr A, Agroudy A, Shehawy E, Shokier A, Ghoneim M. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: FP222]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi93. - PubMed
-
- Neamatalla AH, Bakr MA, Agroudy AE, Shehawy EL, Shokier AA. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: P163]. Transplant International 2007;20(Suppl 2):134. [CENTRAL: CN‐00653762] - PubMed
-
- Nematalla AH, Bakr MA, Agroudy A, Shehawy E, Abdel Rahman ME. Steroid free immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized study (single center experience) [abstract no: PO‐466]. Transplant International 2005;18(Suppl 1):157.
Nott 1985 {published data only}
-
- Griffin PJ, Costa CA, Salaman JR. A controlled trial of steroids in cyclosporine‐treated renal transplant recipients. Transplantation 1987;43(4):505‐8. [MEDLINE: ] - PubMed
-
- Griffin PJ, Gomes da Costa CA, Salaman JR. Renal transplantation without steroids: a controlled clinical trial. Transplantation Proceedings 1986;18(4):797‐8. [EMBASE: 1986223826]
-
- Nott D, Griffin PJ, Salaman JR. Low‐dose steroids do not augment cyclosporine immunosuppression but do diminish cyclosporine nephrotoxicity. Transplantation Proceedings 1985;17(1 II):1289‐90. [EMBASE: 1985078719]
-
- Salaman JR, Gomes Da Costa CA, Griffin PJ. Renal transplantation without steroids. Journal of Pediatrics 1987;111(6 Pt 2):1026‐8. [MEDLINE: ] - PubMed
Park 1994 {published data only}
-
- Kim HC, Chang KJ, Kwon JK, Park SB, Cho WH, Park CH. Long‐term results of cyclosporine monotherapy in renal transplantation. Transplantation Proceedings 1998;30(7):3539‐40. [MEDLINE: ] - PubMed
-
- Park K, Kim ST, Lee SR, Koh YB, Kim HC. A 1‐year prospective randomized study in Korean living donor kidney transplant recipients: comparing cyclosporine monotherapy and cyclosporine/prednisolone during the maintenance phase of immunosuppression. Transplantation Proceedings 1994;26(4):1985‐6. [MEDLINE: ] - PubMed
Pelletier 2006 {published data only}
-
- Akin B, Ferguson RM, Pelletier RP. Five year follow‐up after steroid withdrawal demonstrates no evidence of worsening renal function [abstract]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509047]
-
- Ing SW, Sinnott LT, Davies EA, Pelletier RP. Bone mineral density changes in a prospective, randomized trial of steroid withdrawal in kidney transplant recipients [abstract no: 621]. American Journal of Transplantation 2007;7(Suppl 2):310. [CENTRAL: CN‐00764017]
-
- Pelletier RP, Akin B, Ferguson RM. Prospective, randomized trial of steroid withdrawal in kidney recipients treated with mycophenolate mofetil and cyclosporine. Clinical Transplantation 2006;20(1):10‐8. [MEDLINE: ] - PubMed
-
- Pelletier RP, Davies EA, Elkhammas EA, Bumgardner GL, Henry ML, Ferguson RM. Randomized, prospective trial of prednisone withdrawal in stable renal transplant recipients [abstract]. Transplantation 2000; Vol. 69, issue 8 Suppl:S260. [CENTRAL: CN‐00447152]
Pisani 2001 {published data only}
-
- Coppelli A, Buonomo O, Iaria G, Pisani F, Pollicita S, Rizzello A. Preliminary results of a prospective randomized study of basiliximab and steroid withdrawal in kidney transplantation [abstract no: 1617]. 2001 A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400600]
-
- Pisani F, Buonomo O, Iaria G, Tisone G, Mazzarella V, Pollicita S, et al. Preliminary results of a prospective randomized study of basiliximab in kidney transplantation. Transplantation Proceedings 2001;33(1‐2):2032‐3. [MEDLINE: ] - PubMed
Ponticelli 1997 {published data only}
-
- Aroldi A, Tarantino A, Montagnino G, Cesana B, Cocucci C, Ponticelli C. Effects of three immunosuppressive regimens on vertebral bone density in renal transplant recipients: a prospective study. Transplantation 1997;63(3):380‐6. [MEDLINE: ] - PubMed
-
- Montagnino G, Tarantino A, Maccario M, Elli A, Cesana B, Ponticelli C. Long‐term results with cyclosporine monotherapy in renal transplant patients: a multivariate analysis of risk factors. American Journal of Kidney Diseases 2000;35(6):1135‐43. [MEDLINE: ] - PubMed
-
- Montagnino G, Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, et al. Long‐term results of a randomized study comparing three immunosuppressive schedules with cyclosporine in cadaveric kidney transplantation. Journal of the American Society of Nephrology 2001;12(10):2163‐9. [MEDLINE: ] - PubMed
-
- Ponticelli C. Steroid withdrawal in organ transplant recipients [abstract no: 0351]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00583616]
-
- Ponticelli C, Aroldi A. Osteoporosis after organ transplantation. Lancet 2001;357(9268):1623. [MEDLINE: ] - PubMed
Ratcliffe 1993 {published data only}
-
- Dudley CR, Ratcliffe PJ, et al. Effect of steroid withdrawal on graft function in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 1994;9(11):1672. [CENTRAL: CN‐00261076]
-
- Ratcliffe PJ, Dudley CR, Higgins RM, Firth JD, Smith B, Morris PJ. Randomised controlled trial of steroid withdrawal in renal transplant recipients receiving triple immunosuppression. Lancet 1996;348(9028):643‐8. [MEDLINE: ] - PubMed
-
- Ratcliffe PJ, Firth JD, Higgins RM, Smith B, Gray DW, Morris PJ. Randomized controlled trial of complete steroid withdrawal in renal transplant patients receiving triple immunosuppression. Transplantation Proceedings 1993;25(1 Pt 1):590. [MEDLINE: ] - PubMed
Sandrini 2009 {published data only}
-
- Sandrini S, Setti G, Bossini N, Chiappini R, Valerio F, Mazzola G, et al. Early (fifth day) vs. late (sixth month) steroid withdrawal in renal transplant recipients treated with Neoral plus Rapamune: four‐yr results of a randomized monocenter study. Clinical Transplantation 2010;24(5):669‐77. [MEDLINE: ] - PubMed
-
- Sandrini S, Setti G, Bossini N, Maffei C, Iovinella L, Tognazzi N, et al. Steroid withdrawal five days after renal transplantation allows for the prevention of wound‐healing complications associated with sirolimus therapy. Clinical Transplantation 2009;23(1):16‐22. [MEDLINE: ] - PubMed
Schulak 1989 {published data only}
-
- Hricik DE, Mayes JT, Schulak JA. Independent effects of cyclosporine and prednisone on posttransplant hypercholesterolemia. American Journal of Kidney Diseases 1991;18(3):353‐8. [MEDLINE: ] - PubMed
-
- Hricik DE, Moritz C, Mayes JT, Schulak JA. Association of the absence of steroid therapy with increased cyclosporine blood levels in renal transplant recipients. Transplantation 1990;49(1):221‐3. [MEDLINE: ] - PubMed
-
- Hricik DE, Whalen CC, Lautman J, Bartucci MR, Moir EJ, Mayes JT, et al. Withdrawal of steroids after renal transplantation‐‐clinical predictors of outcome. Transplantation 1992;53(1):41‐5. [MEDLINE: ] - PubMed
-
- Schulak JA, Mayes JT, Moritz CE, Hricik DE. A prospective randomized trial of prednisone versus no prednisone maintenance therapy in cyclosporine‐treated and azathioprine‐treated renal transplant patients. Transplantation 1990;49(2):327‐32. [MEDLINE: ] - PubMed
-
- Schulak JA, Moritz CE, Hricik DE. Renal transplantation without prednisolone: effects of bone marrow tolerance azathioprine. Transplantation Proceedings 1989;21(1 Pt 2):1709‐11. [MEDLINE: ] - PubMed
Smak Gregoor 1999 {published data only}
-
- Roodnat J, Hilbrands LB, Hene RJ, Sevaux RG, Gregoor PJ, Gestel JA, et al. 15 year follow‐up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation [abstract no: BO156]. Transplant International 2013;26(Suppl 2):83‐4. [EMBASE: 71359271]
-
- Roodnat JI, Hilbrands LB, Hene RJ, Sevaux RG, Smak Gregoor PJ, Kal‐van Gestel JA, et al. 15‐year follow‐up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014;98(1):47‐53. [MEDLINE: ] - PubMed
-
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, et al. A prospective randomised study of withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: 18 months follow‐up data [abstract]. American Journal of Transplantation 2001;1(Suppl 1):246. [CENTRAL: CN‐00763745]
-
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hesse CJ, Hilbrands LB, Vos P, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999;68(10):1603‐6. [MEDLINE: ] - PubMed
-
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. Journal of the American Society of Nephrology 2002;13(5):1365‐73. [MEDLINE: ] - PubMed
Sola 2002 {published data only}
-
- Sola E, Alférez MJ, Cabello M, Burgos D, González MM. Low‐dose and rapid steroid withdrawal in renal transplant patients treated with tacrolimus and mycophenolate mofetil. Transplantation Proceedings 2002;34(5):1689‐90. [MEDLINE: ] - PubMed
Stiller 1983 {published data only}
-
- Canadian Transplant Study Group. The requirements for maintenance steroids in cyclosporine‐treated renal transplant recipients [abstract]. Kidney International 1984;25(6):998.
-
- MacDonald AS, Daloze P, Dandavino R, Jindal S, Bear L, Dossetor JB, et al. A randomized study of cyclosporine with and without prednisone in renal allograft recipients. Canadian Transplant Group. Transplantation Proceedings 1987;19(1 Pt 3):1865‐6. [MEDLINE: ] - PubMed
-
- Stiller C. The requirements for maintenance steroids in cyclosporine‐treated renal transplant recipients. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2490‐4. [EMBASE: 1984089082]
THOMAS Study 2002 {published data only}
-
- Boots JM, Ham EC, Christiaans MH, Hooff JP. Risk of adrenal insufficiency with steroid maintenance therapy in renal transplantation. Transplantation Proceedings 2002;34(5):1696‐7. [MEDLINE: ] - PubMed
-
- Budde K, Salmela K, Pascual J, Rigotti P, THOMAS Follow‐up Study Group. Steroid‐withdrawal in tacrolimus‐treated renal transplant recipients: results of a 3‐year follow‐up study [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550470]
-
- Jindal RM, Salmela K, Vanrentergheim Y, Hooff JP, Squifflet JP. Reduction of high cholesterol levels by early withdrawal of steroids from a tacrolimus‐based triple regimen [abstract no: 206]. American Journal of Transplantation 2002;2(Suppl 3):190. [CENTRAL: CN‐00520347]
-
- Pascual J, Hooff JP, Salmela K, Lang P, Rigotti P, Budde K. Three‐year observational follow‐up of a multicenter, randomized trial on tacrolimus‐based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 2006;82(1):55‐61. [MEDLINE: ] - PubMed
-
- Pascual J, Vanrenterghem Y, Hooff JP, Squifflet JP, Salmela K, Rigotti P. Safe withdrawal of MMF or steroids following 3‐months of tacrolimus triple therapy: results of a large, prospective, multicentre study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416426]
Vincenti 2003a {published data only}
-
- Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, et al. Health‐related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney International 2003;63(6):2309‐16. [MEDLINE: ] - PubMed
-
- Topp KS, Painter PL, Walcott S, Krasnoff JB, Adey D, Sakkas GK, et al. Alterations in skeletal muscle structure are minimized with steroid withdrawal after renal transplantation. Transplantation 2003;76(4):667‐73. [MEDLINE: ] - PubMed
-
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Neylan J, Roza A, et al. A multicenter randomized trial of rapid steroid withdrawal vs standard steroid treatment in patients treated with Simulect, Neoral and Cellcept for the prevention of acute rejection in renal transplantation [abstract no: 583]. Transplantation 1999;67(7):S152. [CENTRAL: CN‐00403005]
-
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Neylan J, Roza A, et al. Rapid steroid withdrawal versus standard steroid therapy in patients treated with basiliximab, cyclosporine, and mycophenolate mofetil for the prevention of acute rejection in renal transplantation. Transplantation Proceedings 2001;33(1‐2):1011‐2. [MEDLINE: ] - PubMed
-
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Roza A. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. American Journal of Transplantation 2003;3(3):306‐11. [MEDLINE: ] - PubMed
Woodle 2005 {published data only}
-
- Alloway R, Woodle ES, Gaber AO, Pirsch J, Shihab F, Veldhuisen P, et al. Multivariate analysis of risk factors for acute rejection with early (7 day) corticosteroid withdrawal: results from a randomized, double blind, placebo‐controlled trial [abstract no: 567]. American Journal of Transplantation 2006;6(Suppl 2):257.
-
- Gaber AO, Moore LW, Alloway RR, Woodle ES, Pirsch J, Shihab F, et al. Acute rejection characteristics from a prospective, randomized, double‐blind, placebo‐controlled multicenter trial of early corticosteroid withdrawal. Transplantation 2013;95(4):573‐9. [MEDLINE: ] - PubMed
-
- Gaber AO, Moore LW, Pirsch J, Shihab F, Woodle ES, Astellas Steroid Withdrawal Study Group. Characteristics of rejection in renal allografts following early corticosteroid withdrawal in a randomized controlled clinical trial: results of 3 year followup [abstract no: 330]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00764094]
-
- Lin S, Henning AK, Akhlaghi F, Reisfield R, Vergara‐Silva A, First MR. Interleukin‐2 receptor antagonist therapy leads to increased tacrolimus levels after kidney transplantation. Therapeutic Drug Monitoring 2015;37(2):206‐13. [MEDLINE: ] - PubMed
-
- Pirsch J, Woodle ES, Shihab F, Gaber AO, Veldhuisen P, Gao J, et al. Effect of steroid withdrawal on new onset diabetes after transplant: results of a randomized double‐blind placebo controlled trial [abstract no: 856]. American Journal of Transplantation 2006;6(Suppl 2):355.
Zhu 2008a {published data only}
-
- Zhu QG, Zhao YK, Liu W, Luo H, Qiu Y, Gao ZZ. Two‐year observation of a randomized trial on tacrolimus‐based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplantation. Chinese Medical Sciences Journal 2008;23(4):244‐8. [MEDLINE: ] - PubMed
References to studies excluded from this review
Alexander 2006 {published data only}
-
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transplant International 2006;19(4):295‐302. [MEDLINE: ] - PubMed
Anil Kumar 2005 {published data only}
-
- Anil Kumar MS, Fyfe B, Sierka D, Heifets M, Saeed MI, Parikh MH. Comparison of efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) as adjunct to calcineurin inhibitor (CNIi) based steroid free immunosuppression in kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509056]
-
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80(6):807‐14. [MEDLINE: ] - PubMed
-
- Anil Kumar MS, Heifets M, Fyfe B, Sierka D, Saeed MI, Parekh M, et al. A prospective randomized study to compare the efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) monitored by protocol biopsies in tacrolimus (TAC) based steroid free immunosuppression [abstract]. American Journal of Transplantation 2004;4(Suppl 8):216. [CENTRAL: CN‐00509296]
-
- Kumar A, Lee D, Xiao SG, Moritz MJ, Fyfe B, Heifets M, et al. Comparison of tacrolimus (FK506) and sirolimus (SRL) combination with FK506 and mycophenolate mofetil (MMF) in kidney transplant recipients with steroid avoidance [abstract]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446223]
Axelrod 2005 {published data only}
-
- Axelrod D, Leventhal JR, Gallon LG, Parker MA, Kaufman DB. Reduction of CMV disease with steroid‐free immunosuppression in simultaneous pancreas‐kidney transplant recipients. American Journal of Transplantation 2005;5(6):1423‐9. [MEDLINE: ] - PubMed
Berney 2004 {published data only}
-
- Berney T, Bucher P, Mathe Z, Andres A, Bosco D, Mage R, et al. Islet of langerhans allogeneic transplantation at the University of Geneva in the steroid free era in islet after kidney and simultaneous islet‐kidney transplantations. Transplantation Proceedings 2004;36(4):1121‐2. [MEDLINE: ] - PubMed
Birkeland 1998b {published data only}
-
- Birkeland SA, Larsen KE, Rohr N. Pediatric renal transplantation without steroids. Pediatric Nephrology 1998;12(2):87‐92. [MEDLINE: ] - PubMed
Birkeland 2002 {published data only}
-
- Birkeland SA, Beck‐Nielsen H, Rohr N, Bertuzzi F, Secchi A, Shapiro J, et al. Steroid‐free immunosuppression in kidney‐islet transplantation: a long‐term follow‐up. Transplantation 2002;73(9):1527. [MEDLINE: ] - PubMed
Budde 2001 {published data only}
-
- Budde K, Diekmann F, Fritsche L, Geissler S, Hallebach G, Neumayer H. Steroid withdrawal in long‐term cyclosporine treated patients using mycophenolate mofetil: a prospective randomized pilot study [abstract no: 1233]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00644340] - PubMed
-
- Budde K, Fritsche L, Geissler S, Hallebach G, Diekmann F, Mai I, et al. Steroid withdrawal in long‐term cyclosporine A treated patients using mycophenolate mofetil: a prospective randomized pilot study. Transplantation Proceedings 2001;33(7‐8):3250‐2. [MEDLINE: ] - PubMed
-
- Budde K, Geissler S, Hallebach G, Fritsche L, Waiser J, Neumayer H. Prospective randomized trial of steroid withdrawal in mycophenolate mofetil (MMF) and cyclosporine (CYA) treated patients (PTS) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):668A. [CENTRAL: CN‐00444574]
-
- Budde K, Geissler S, Hallebach G, Waiser J, Fritsche L, Bohler T, et al. Prospective randomized pilot study of steroid withdrawal with mycophenolate mofetil in long‐term cyclosporine‐treated patients: 4‐year follow‐up. Transplantation Proceedings 2002;34(5):1703‐5. [MEDLINE: ] - PubMed
CAMPASIA Study 2005 {published data only}
-
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] - PubMed
-
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosporine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538]
-
- Vathsala A, Campasia Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl):A215. [CENTRAL: CN‐00583367]
-
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. CAMPASIA: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539]
-
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Lymphocyte recovery after depletion by alemtuzumab in renal transplant recipients: impact on outcome [abstract no: 1015]. American Journal of Transplantation 2005;5(Suppl 11):415. [CENTRAL: CN‐00644284]
CARMEN Study 2005 {published data only}
-
- Budde K, Neumayer HH, Rostaing L, Catarovich D, Mourad G, Rigotti P, et al. Steroid‐free immunosuppression with daclizumab, tacrolimus and MMF is efficacious and improves cholesterol, glucose and bone mineral density ‐ the CARMEN study [abstract]. Transplantation 2004;78(2 Suppl):168. [CENTRAL: CN‐00509111]
-
- Cantarovich D, Rostaing L, Mourad G, Neumayer HH, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. The combination of Daclizumab, Tacrolimus, and MMF is an effective and safe steroid‐free immunosuppressive regimen after renal transplantation. Results of a large multicentre trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788. [CENTRAL: CN‐00444672]
-
- Kramer BK, Kruger B, Mack M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐91. [MEDLINE: ] - PubMed
-
- Mourad G, Rostaing L, Cantarovich D, Neumayer H, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. Immunosuppression without steroids: daclizumab/tacrolimus/MMF vs. tacrolimus/MMF/steroids in renal transplantation [abstract no: 12]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003. [CENTRAL: CN‐00653705]
-
- Pascual J, Rigotti P, Vialtel P, Sanchez‐Fructuoso A, Escuin F, Bone Mineral Density Study Group. Immunosuppression without steroids: a daclizumab, tacrolimus and MMF regimen prevents loss of bone mass following renal transplantation [abstract no: 369]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003. [CENTRAL: CN‐00653764]
Citterio 2002 {published data only}
-
- Citterio F, Baldan N, Tondolo E, Marchini F, Castagneto M, Rigotti P. Medium term results of steroid withdrawal in tacrolimus treated renal transplant recipients [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550659]
-
- Citterio F, Baldan N, Tondolo V, Marchini F, Romagnoli J, Furian L, et al. Five years prospective study of steroid withdrawal in renal transplant recipients [abstract no: 340]. American Journal of Transplantation 2005;5(Suppl 11):242. [CENTRAL: CN‐00644339]
-
- Citterio F, Rigotti P, Scata MC, Baldan N, Marchini F, Castagneto M. Steroid withdrawal in renal transplant patients immunosuppressed with tacrolimus [abstract no: 135]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00415437] - PubMed
-
- Citterio F, Rigotti P, Scata MC, Romagnoli J, Baldan N, Marchini F, et al. Steroid withdrawal from tacrolimus‐based therapy in renal transplant patients. Transplantation Proceedings 2002;34(5):1707‐8. [MEDLINE: ] - PubMed
CORRETA Study 2008 {published data only}
-
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. CORRETA trial (corticosteroid reduction with tacrolimus): prospective Brazilian multicenter, randomized trial of early corticosteroid reduction vs regular corticosteroid dosage maintenance on a tacrolimus (Prograf®) and mycophenolate mofetil (Cellcept®) immunosuppression regimen in kidney transplant recipients ‐ interim analysis [abstract no: P146]. Transplant International 2007;20(Suppl 2):130‐1. [CENTRAL: CN‐00724889] - PubMed
-
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. Corticosteroid reduction with tacrolimus (CORRETA) TRIAL: a prospective Brazilian multicenter, randomized trial of early corticosteroid reduction versus regular corticosteroid dosage maintenance on a tacrolimus (Prograf) and mycophenolate mofetil (Cellcept) immunosuppression regimen in kidney transplant recipients: interim analysis. Transplantation Proceedings 2008;40(3):689‐92. [MEDLINE: ] - PubMed
-
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. Randomized trial of early corticosteroid reduction vs. regular‐dose corticosteroid maintenance in combination with tacrolimus and mycophenolate mofetil in living donor kidney transplant recipients: the Brazilian CORRETA trial. Clinical Transplantation 2010;24(4):E109‐15. [MEDLINE: ] - PubMed
Curtis 1982 {published data only}
-
- Curtis JJ, Galla JH, Woodford SY, Lucas BA, Luke RG. Effect of alternate‐day prednisone on plasma lipids in renal transplant recipients. Kidney International 1982;22(1):42‐7. [MEDLINE: ] - PubMed
Daniel 1985 {published data only}
-
- Daniel V, Opelz G, Dreikorn K. Lymphocyte subpopulations in kidney transplant patients with different types of immunosuppression. Transplantation Proceedings 1985;17(6):2254‐7. [EMBASE: 1986058424]
-
- Dreikorn K, Horsch R, Rohl L. A randomized trial with different immunosuppressive regimens after renal transplantation. Transplantation Proceedings 1985;17(6):2663‐5. [EMBASE: 1986055110]
De Backer 1992 {published data only}
-
- Backer D, Abramowicz D, Goldman M, Pauw L, Viseur P, Vanherweghem JL, et al. High or low dose steroid therapy for acute renal transplant rejection after prophylactic OKT3 treatment: a prospective randomized study. Transplant International 1992;5 Suppl 1:S437‐9. [MEDLINE: ] - PubMed
Delucchi 2006 {published data only}
-
- Delucchi B A, Valenzuela A M, Ferrario B M, Lillo D AM, Guerrero G JL, Rodriguez S E, et al. Early steroid withdrawal in pediatric renal transplantation [Retiro precoz de esteroides en la inmunosupresion del trasplante renal pediatrico]. Revista Medica de Chile 2006;134(11):1393‐401. [MEDLINE: ] - PubMed
de Sandes Freitas 2011 {published data only}
-
- Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. International Urology & Nephrology 2011;43(4):1221‐8. [MEDLINE: ] - PubMed
ECSEL Study 2008 {published data only}
-
- Smith MP, Newstead CG, Ahmad N, Lewington AJ, Tibble S, Lodge JP, et al. Poor tolerance of sirolimus in a steroid avoidance regimen for renal transplantation. Transplantation 2008;85(4):636‐9. [MEDLINE: ] - PubMed
-
- Welberry‐Smith MP, Gone K, Tibble S, Littler D, Newstead CG, et al. Poor tolerance of sirolimus in a steroid avoidance regime [abstract no: P37]. British Transplantation Society (BTS). 9th Annual Congress; 2006 Mar 29‐31; Edinburgh, UK. 2006.
Hibbs 2010 {published data only}
-
- Hibbs J, Hamdallah O, Cannon R, Ravindra K, Ouseph R, Gleason J, et al. Alemtuzumab induction with rapid corticosteroid elimination is associated with comparable outcomes in high immunologic risk and non high risk renal transplant recipients [abstract no: 976]. American Journal of Transplantation 2010;10(Suppl 4):322. [EMBASE: 70464352]
Hilbrands 1993 {published data only}
-
- Hilbrands LB, Demacker PN, Hoitsma AJ. Cyclosporin and serum lipids in renal transplant recipients. Lancet 1993;341(8847):765‐6. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (APO)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene KA. Randomized, prospective trial of cyclosporine monotherapy versus azathioprine‐prednisone from three months after renal transplantation. Transplantation 1996;61(7):1038‐46. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene RA. Costs of drugs used after renal transplantation. Transplant International 1996;9 Suppl 1:S399‐402. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene RA. Effect of immunosuppressive therapy on quality of life after renal transplantation [abstract]. Journal of the American Society of Nephrology 1994;5(3):1011. [CENTRAL: CN‐00615875]
Hodson 1989 {published data only}
-
- Hodson EM, Knight JF, Sheil AG, Roy LP. Cyclosporin A as sole immunosuppressive agent for renal transplantation in children: effect on catch‐up growth. Transplantation Proceedings 1989;21(1 Pt 2):1687‐92. [MEDLINE: ] - PubMed
Hricik 1993a {published data only}
-
- Hricik DE, Schulak JA. Metabolic effects of steroid withdrawal in adult renal transplant recipients. Kidney International ‐ Supplement 1993;43:S26‐9. [MEDLINE: ] - PubMed
Hricik 1993b {published data only}
-
- Hricik DE, O'Toole MA, Schulak JA, Herson J. Steroid‐free immunosuppression in cyclosporine‐treated renal transplant recipients: a meta‐analysis. Journal of the American Society of Nephrology 1993;4(6):1300‐5. [MEDLINE: ] - PubMed
John 2005 {published data only}
-
- John E, Lumpaopang A, Oberholzer J, Testa G, Sankary H, Benedetti E. Superior outcomes in growth and renal function with early steroid discontinuation in pediatric kidney transplantation: 1 1/2 years follow‐up [abstract no: 1329]. American Journal of Transplantation 2005;5(Suppl 11):494.
Juarez 2006 {published data only}
-
- Juarez FJ, Barrios Y, Cano L, Lopez E, Martinez J, Limones M, et al. A randomized trial comparing two corticosteroid regimens combined with mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation Proceedings 2006;38(9):2866‐8. [MEDLINE: ] - PubMed
Kim 2004 {published data only}
-
- Kim B, Huh W, Baek HJ, Lim YH, Yeo HM, Kim JA, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients [abstract no: SU‐PO523]. Journal of the American Society of Nephrology 2003;14(Nov):648A. [CENTRAL: CN‐00626065]
-
- Kim B, Huh W, Kim MO, Yeo HM, Kim HJ, Kim JA, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients. Korean Journal of Nephrology 2004;23(5):785‐92. [CENTRAL: CN‐01044957] - PubMed
-
- Kim SJ, Lee KW, Lee DS, Lee HH, Lee SK, Kim B, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients. Transplantation Proceedings 2004;36(7):2098‐100. [MEDLINE: ] - PubMed
Kim 2005 {published data only}
-
- Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatric Transplantation 2005;9(2):162‐9. [MEDLINE: ] - PubMed
Lehmann 2004 {published data only}
-
- Lehmann R, Weber M, Berthold P, Zullig R, Pfammatter T, Moritz W, et al. Successful simultaneous islet‐kidney transplantation using a steroid‐free immunosuppression: two‐year follow‐up. American Journal of Transplantation 2004;4(7):1117‐23. [MEDLINE: ] - PubMed
Li 2011a {published data only}
-
- Li S, Wang W, Hu X, Ren L, Yin H, Yang X, et al. The effects of early rapid corticosteroid reduction on cell‐mediated immunity in kidney transplant recipients. Transplant Immunology 2011;24(2):127‐30. [MEDLINE: ] - PubMed
-
- Li SH, Wang W, Hu XP, Yin H, Ren L, Yang XY, et al. Monitoring immune function after rapid corticosteroid reduction in kidney transplant recipients. Chinese Medical Journal 2011;124(5):679‐82. [MEDLINE: ] - PubMed
Morris 1982 {published data only}
-
- Morris PJ, Chan L, French ME, Ting A. Low dose oral prednisolone in renal transplantation. Lancet 1982;1(8271):525‐7. [MEDLINE: ] - PubMed
MYSS Study 2004 {published data only}
-
- Gotti E, Perico N, Gaspari F, Cattaneo D, Lesti MD, Ruggenenti P, et al. Blood cyclosporine level soon after kidney transplantation is a major determinant of rejection: insights from the Mycophenolate Steroid‐Sparing Trial. Transplantation Proceedings 2005;37(5):2037‐40. [MEDLINE: ] - PubMed
-
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation blood cyclosporine levels soon after surgery act as a major determinant of rejection: insights from the MY.S.S. trial. Kidney International 2004;65(3):1084‐90. [MEDLINE: ] - PubMed
-
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation low blood cyclosporine levels soon after surgery is a determinant of rejection: insights from the MY.S.S. trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):11A. [CENTRAL: CN‐00601980]
-
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow‐up randomized, controlled clinical trial. Journal of the American Society of Nephrology 2007;18(6):1973‐85. [MEDLINE: ] - PubMed
-
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene‐Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364(9433):503‐12. [MEDLINE: ] - PubMed
NCT00089947 {published data only}
-
- NCT00089947. Randomized, prospective, phase 2 study comparing thymoglobulin in a rapid discontinuation of corticosteroids protocol with standard corticosteroid therapy in living donor renal transplantation using mycophenolate mofetil and tacrolimus maintenance therapy. www.clinicaltrials.gov/ct2/show/NCT00089947 (accessed 15 February 2016).
Nori 2008 {published data only}
-
- Nori US, Pesavento TE, Davies EA, Visger J, Miller BS, Ferguson RM. Randomized, prospective prednisone (P) withdrawal trial in kidney transplant patients treated with sirolimus (S) vs microemulsified cyclosporine (CsA) based regimens [abstract no: 458]. American Journal of Transplantation 2008;8(Suppl 2):301. [CENTRAL: CN‐00725014]
Paczek 2003a {published data only}
-
- Paczek L, Wlodarczyk Z, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Absence of rejection and stable serum creatinine are excellent criteria for steroid‐withdrawal in kidney transplant patients receiving tacrolimus treatment [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):787‐8. [CENTRAL: CN‐00447076]
Papadakis 1982 {published data only}
-
- Papadakis JT, Bewick M, Cameron JS, Rudge C, Ogg CS, Brown CB, et al. Low dose steroids in renal transplantation. Lancet 1982;1(8277):916‐7. [MEDLINE: ] - PubMed
Reed 1991 {published data only}
-
- Reed A, Pirsch JD, Armbrust MJ, Burlingham WJ, Knechtle SJ, D'Alessandro AM, et al. A comparison of donor‐specific and random transfusions in living‐related renal transplantation and their effect on steroid withdrawal. Transplantation Proceedings 1991 Feb;23(1 Pt 2):1321‐2. [MEDLINE: ] - PubMed
Remport 2001 {published data only}
-
- Remport A, Sasvari I, Borka P, Toronyi E, Sarvary E, Weszelits W, et al. Comparative analysis of mycophenolate‐mofetil‐cyclosporin immunosuppression of kidney transplantation recipients with two different corticosteroid doses and conventional cyclosporin‐corticosteroid therapy [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00447378]
-
- Remport A, Sasvari I, Borka P, Toronyi E, Sarvary E, Weszelits W, et al. Evaluation of the effect of different corticosteroid doses in combination with mycophenolate‐mofetil‐cyclosporin immunosuppression in kidney transplanted recipients [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A256. [CENTRAL: CN‐00461586]
-
- Remport A, Sasvari I, Toronyi E, Borka P, Lazar N, Jaray J, et al. Mycofenolate mofetil‐cyclosporine immunosuppression of kidney transplantation recipients with two different corticosteroid doses. Transplantation Proceedings 2001;33(3):2302‐3. [MEDLINE: ] - PubMed
Robertson 1980 {published data only}
Sarwal 2012 {published data only}
-
- Kambham N, Naesens M, Sigdel T, Waskerwitz J, Salvatierra O, Sarwal M. A protocol biopsy analysis from an NIH multicenter pediatric renal transplant trial reveals no adverse effect of steroid avoidance on the histological evolution of chronic graft injury [abstract no: LB01]. American Journal of Transplantation 2008;8(Suppl 2):334.
-
- Naesens M, Kambham N, Sigdel T, Waskerwitz J, Salvatierra O, Sarwal M. A protocol biopsy analysis from an NIH multicenter pediatric renal transplant trial revels no adverse effect of steroid avoidance on the histological evolution of chronic graft injury [abstract no: 54]. Transplantation 2008;86(2S):18.
-
- Naesens M, Salvatierra O, Benfield M, Ettenger RB, Dharnidharka V, Harmon W, et al. Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation. American Journal of Transplantation 2012;12(10):2730‐43. [MEDLINE: ] - PMC - PubMed
-
- Sarwal M, Benfield M, Ettenger R, Dharnidharka V, Mathias R, McDonald R, et al. One year results of a prospective, randomized, multicenter trial of steroid avoidance in pediatric renal transplantation [abstract no: 52]. American Journal of Transplantation 2008;8(Suppl 2):192.
SENIOR Study 2009 {published data only}
-
- Andres A, Budde K, Clavien PA, Becker T, Kessler M, Pisarski P, et al. A randomized trial comparing renal function in older kidney transplant patients following delayed versus immediate tacrolimus administration. Transplantation 2009;88(9):1101‐8. [MEDLINE: ] - PubMed
Shapiro 1993 {published data only}
-
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Gitsch HA, McCauley J, et al. The outcome after steroid withdrawal in renal transplant patients receiving tacrolimus‐based immunosuppression [abstract no: 188]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:131. [CENTRAL: CN‐00509473]
Silverstein 2005 {published data only}
-
- Silverstein DM, Aviles DH, LeBlanc PM, Jung FF, Vehaskari VM. Results of one‐year follow‐up of steroid‐free immunosuppression in pediatric renal transplant patients. Pediatric Transplantation 2005;9(5):589‐97. [MEDLINE: ] - PubMed
SOCRATES Study 2014 {published data only}
-
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103.
Tarantino 1991 {published data only}
-
- Tarantino A, Aroldi A, Stucchi L, Montagnino G, Mascaretti L, Vegeto A, et al. A randomized prospective trial comparing cyclosporine monotherapy with triple‐drug therapy in renal transplantation. Transplantation 1991;52(1):53‐7. [MEDLINE: ] - PubMed
Teplan 2003 {published data only}
-
- Teplan V, Schuck O, Stollova M, Vitko S. Obesity and hyperhomocysteinaemia after kidney transplantation. Nephrology Dialysis Transplantation 2003;18 Suppl 5:v71‐3. [MEDLINE: ] - PubMed
ter Meulen 2002 {published data only}
-
- Hendrikx TK, Klepper M, Ijzermans J, Weimar W, Baan CC. Clinical rejection and persistent immune regulation in kidney transplant patients. Transplant Immunology 2009;21(3):129‐35. [MEDLINE: ] - PubMed
-
- Hesselink DA, Ngyuen H, Wabbijn M, Smak Gregoor PJH, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids [abstract]. American Journal of Transplantation 2003;3(Suppl 5):482. - PMC - PubMed
-
- ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin‐2 receptor alpha after treatment with daclizumab. Kidney International 2003;64(2):697‐703. [MEDLINE: ] - PubMed
-
- ter Meulen CG, Hilbrands LB, Bergh JP, Hermus AR, Hoitsma AJ. The influence of corticosteroids on quantitative ultrasound parameters of the calcaneus in the 1st year after renal transplantation. Osteoporosis International 2005;16(3):255‐62. [MEDLINE: ] - PubMed
TRIMS Study 2010 {published data only}
-
- Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC, TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with Thymoglobulin in living‐donor kidney transplantation. Clinical Transplantation 2010;24(1):73‐83. [MEDLINE: ] - PubMed
-
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter comparative study evaluating a thymoglobulin‐based early corticosteroid cessation regime in renal transplantation [abstract no: 673]. American Journal of Transplantation 2006;6(Suppl 2):294. [CENTRAL: CN‐00716028]
-
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS) [abstract no: 1632]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00716027]
TWIST Study 2010 {published data only}
-
- Billing H, Hoecker B, Fichtner A, Damme‐Lombaerts R, Friman S, Jaray J, et al. Single nucleotide polymorphism of CYP3A5 influences the exposure to tacrolimus in pediatric renal transplant recipients: A pharmacogenetic substudy of the TWIST trial [abstract]. Transplantation 2014;98(Suppl 1):147. [EMBASE: 71543993]
-
- Billing H, Sander A, Susal C, Ovens J, Feneberg R, Hocker B, et al. Soluble CD30 and ELISA‐detected human leukocyte antigen antibodies for the prediction of acute rejection in pediatric renal transplant recipients. Transplant International 2013 Mar;26(3):331‐8. [MEDLINE: ] - PubMed
-
- Feneberg R. Single nucleotide polymorphisms of CYP3A5, but not of other genes, influence the exposure to tacrolimus in paediatric renal transplant recipients: a pharmacognetic substudy of the Twist Study [abstract no: SAT‐M‐130]. Transplantation Society of Australia & New Zealand (TSANZ). 27th Annual Meeting; 2009 Jun 17‐19; Canberra (ACT). 2009:121.
-
- Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. American Journal of Transplantation 2010;10(4):828‐36. [MEDLINE: ] - PubMed
-
- Trompeter RS, Grenda R, Watson A. Improved growth in pediatric kidney recipients after early steroid withdrawal: daclizumab, tacrolimus (TAC) and mycophenolate mofetil (MMF) versus TAC, MMF and steroids (TWIST Study) [abstract no: 604]. American Journal of Transplantation 2009;9(Suppl 2):365. [EMBASE: 70010477]
Weimert 2008 {published data only}
-
- Weimert N, Alloway R, Vinks A, Rike A, Young S, Cardi M, et al. A 12‐month, prospective, randomized, single center, open label pilot study to evaluate the safety and efficacy of Myfortic® in combination with tacrolimus and thymoglobulin® in early corticosteroid withdrawal [abstract no: 102]. Transplantation 2008;86(2 Suppl):36.
References to studies awaiting assessment
Newstead 1989 {published data only}
-
- Newstead C, Moore R, et al. Renal transplant function after steroid withdrawal from triple immunosuppression [abstract]. Nephrology Dialysis Transplantation 1989;4:518. [CENTRAL: CN‐00260463]
Additional references
ANZDATA 2012
-
- Clayton P, Campbell S, Chadban S, McDonald S, Hurst K. ANZDATA Registry Report 2012. Adelaide, South Australia: Australia and New Zealand Dialysis and Transplant Registry, 2012.
Basadonna 1993
-
- Basadonna GP, Matas AJ, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, et al. Early versus late acute renal allograft rejection: Impact on chronic rejection. Transplantation 1993;55(5):993‐5. [MEDLINE: ] - PubMed
Coutinho 2011
Cuervo 2003
Czock 2005
-
- Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clinical Pharmacokinetics 2005;44(1):61‐98. [MEDLINE: ] - PubMed
Da Silva 2006
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Publishing Group, 2001:285–312.
ERA‐EDTA 2013
-
- ERA‐EDTA Registry. ERA‐EDTA Registry Annual Report 2011. Amsterdam: Academic Medical Center, Department of Medical Informatics, 2013.
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
Hricik 1993
-
- Hricik DE, O'Toole MA, Schulak JA, Herson J. Steroid‐free immunosuppression in cyclosporine‐treated renal transplant recipients: a meta‐analysis. Journal of the American Society of Nephrology 1993;4(6):1300‐5. [MEDLINE: ] - PubMed
Hricik 2002
-
- Hricik DE. Steroid‐free immunosuppression in kidney transplantation: an editorial review. American Journal of Transplantation 2002;2(1):19‐24. [MEDLINE: ] - PubMed
Juni 1999
-
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054–60. [MEDLINE: ] - PubMed
Kasiske 2000
-
- Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta‐analysis of immunosuppression withdrawal trials in renal transplantation. Journal of the American Society of Nephrology 2000;11(10):1910‐7. [MEDLINE: ] - PubMed
Knight 2010
-
- Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta‐analysis. Transplantation 2010;89(1):1‐14. [MEDLINE: ] - PubMed
Massy 1996
-
- Massy ZA, Guijarro C, Kasiske BL. Clinical predictors of chronic renal allograft rejection. Kidney International ‐ Supplement 1996;52:S85‐8. [MEDLINE: ] - PubMed
Matas 2005
-
- Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, et al. Prednisone‐free maintenance immunosuppression‐a 5‐year experience. American Journal of Transplantation 2005;5(10):2473‐8. [MEDLINE: ] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M. Does the quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] - PubMed
Opelz 2005
-
- Opelz G, Dohler B, Laux G, Collaborative Transplant Study. Long‐term prospective study of steroid withdrawal in kidney and heart transplant recipients. American Journal of Transplantation 2005;5(4 Pt 1):720‐8. [MEDLINE: ] - PubMed
OPTN/SRTR 2014
-
- Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, United Network for Organ Sharing. 2012 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994‐2013. Ann Arbor, USA: HHS/HRSA/OSP/DOT and UNOS, 2012.
Pascual 2002
-
- Pascual M, Theruvath T, Kawai T, Tolkoff‐Rubin N, Cosimi AB. Strategies to improve long‐term outcomes after renal transplantation. New England Journal of Medicine 2002;346(8):580‐90. [MEDLINE: ] - PubMed
Pascual 2004
-
- Pascual J, Quereda C, Zamora J, Hernandez D. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta‐analysis of randomized controlled trials. Transplantation 2004;78(10):1548‐56. [MEDLINE: ] - PubMed
Pascual 2012
-
- Pascual J, Royuela A, Galeano C, Crespo M, Zamora J. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrology Dialysis Transplantation 2012;27(2):825‐32. [MEDLINE: ] - PubMed
Patel 2001
-
- Patel S, Kwan JT, McCloskey E, McGee G, Thomas G, Johnson D, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. Journal of Bone & Mineral Research 2001;16(10):1863‐70. [MEDLINE: ] - PubMed
Prasad 2003
-
- Prasad GV, Nash MM, McFarlane PA, Zaltzman JS. Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clinical Transplantation 2003;17(2):135‐9. [MEDLINE: ] - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] - PubMed
Tunis 2003
-
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials; increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290(12):1624‐32. [MEDLINE: ] - PubMed
References to other published versions of this review
Pascual 2006
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

