L-MYC Expression Maintains Self-Renewal and Prolongs Multipotency of Primary Human Neural Stem Cells
- PMID: 27546534
- PMCID: PMC5031988
- DOI: 10.1016/j.stemcr.2016.07.013
L-MYC Expression Maintains Self-Renewal and Prolongs Multipotency of Primary Human Neural Stem Cells
Abstract
Pre-clinical studies indicate that neural stem cells (NSCs) can limit or reverse CNS damage through direct cell replacement, promotion of regeneration, or delivery of therapeutic agents. Immortalized NSC lines are in growing demand due to the inherent limitations of adult patient-derived NSCs, including availability, expandability, potential for genetic modifications, and costs. Here, we describe the generation and characterization of a new human fetal NSC line, immortalized by transduction with L-MYC (LM-NSC008) that in vitro displays both self-renewal and multipotent differentiation into neurons, oligodendrocytes, and astrocytes. These LM-NSC008 cells were non-tumorigenic in vivo, and migrated to orthotopic glioma xenografts in immunodeficient mice. When administered intranasally, LM-NSC008 distributed specifically to sites of traumatic brain injury (TBI). These data support the therapeutic development of immortalized LM-NSC008 cells for allogeneic use in TBI and other CNS diseases.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

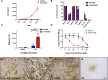
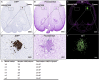

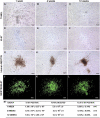

References
-
- Arrowsmith C.H., Bountra C., Fish P.V., Lee K., Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discov. 2012;11:384–400. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

