Femilis(®) 60 Levonorgestrel-Releasing Intrauterine System-A Review of 10 Years of Clinical Experience
- PMID: 27547046
- PMCID: PMC4979586
- DOI: 10.4137/CMRH.S40087
Femilis(®) 60 Levonorgestrel-Releasing Intrauterine System-A Review of 10 Years of Clinical Experience
Abstract
Objective: The aim of this study was to update the clinical experience with the Femilis® 60 levonorgestrel-releasing intrauterine system (LNG-IUS), now up to 10 years in parous and nulliparous women, particularly with regard to ease and safety of insertion, contraceptive performance, retention, acceptability, continuation of use, impact on menstrual blood loss (MBL), and duration of action.
Study design: Using the Femilis® 60 LNG-IUS releasing 20 µg of levonorgestrel/day, the following studies were conducted: an open, prospective noncomparative contraceptive study, an MBL study, a perimenopausal study, a study for the treatment of endometrial hyperplasia, and early cancer of the uterus, a residue study.
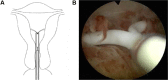
Results: A total of 599 Femilis LNG-IUS were inserted in various clinical trials, the majority for contraceptive purposes. The total exposure in the first and second contraceptive studies, covering 558 parous and nulliparous women, was 32,717 woman-months. Femilis has high contraceptive effectiveness as only one pregnancy occurred. Expulsion of the LNG-IUS was rare with only two total and no partial expulsions (stem protruding through the cervical canal) occurred. Femilis was well tolerated, with continuation rates remaining high. Several MBL studies were conducted, totaling 80 heavy and normal menstrual bleeders, using the pictorial bleeding assessment chart method or the quantitative alkaline hematin technique. Virtually all women responded well with strongly reduced menstrual bleeding. Amenorrhea rates were high, up to 80% after three months, and ferritin levels simultaneously increased significantly. The Femilis LNG-IUS was tested in 104 symptomatic perimenopausal women for seamless transition to and through menopause, adding estrogen therapy when required. Patient tolerability appeared high as >80% requested a second and a third LNG-IUS. Twenty women presenting with nonatypical and atypical hyperplasia and one woman presenting with early endometrial carcinoma were treated with Femilis LNG-IUS. All histology specimens showed full regression, and patients remained in remission without signs of hyperplasia or cancer at yearly and ongoing follow-up examinations up to 10 years. Residual content of LNG was measured in 37 women having the Femilis LNG-IUS for up to 10 years. In 10 of the 102 women who had the Femilis 60 in situ for 10 years between 20% and 30% of the original 60 mg was recovered confirming the long duration of action of the Femilis 60 LNG-IUS.
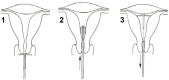
Conclusion: These studies suggest that the Femilis 60 LNG-IUS releasing 20 µg of LNG/day is an effective, well-tolerated, and well-retained contraceptive both in parous and in nulliparous women. The design of the LNG-IUS, with flexible transverse arm(s) length of 28 mm, allows for a simplification of the insertion technique and training requirements facilitating the use by nonspecialist providers in either developed or developing countries. For nulliparous women, additional evaluation of devices with a 24 mm transverse arm(s), as it relates to tolerability, retention, and continuation of use, still needs to be undertaken.
Keywords: T-shaped intrauterine system; contraception; endometrial cancer; heavy menstrual bleeding; hormone replacement therapy; hyperplasia; levonorgestrel.
Figures


Similar articles
-
Ease of insertion, contraceptive efficacy and safety of new T-shaped levonorgestrel-releasing intrauterine systems.Contraception. 2005 Jun;71(6):465-9. doi: 10.1016/j.contraception.2004.12.021. Contraception. 2005. PMID: 15914138
-
The Femilis LNG-IUS: contraceptive performance-an interim analysis.Eur J Contracept Reprod Health Care. 2009 Apr;14(2):103-10. doi: 10.1080/13625180802706059. Eur J Contracept Reprod Health Care. 2009. PMID: 19340705
-
Results of a 5-year contraceptive trial in parous and nulliparous women with a new LNG-IUS.Gynecol Endocrinol. 2017 Mar;33(3):223-226. doi: 10.1080/09513590.2016.1276164. Epub 2017 Jan 13. Gynecol Endocrinol. 2017. PMID: 28084114
-
A risk-benefit assessment of the levonorgestrel-releasing intrauterine system.Drug Saf. 1996 Dec;15(6):430-40. doi: 10.2165/00002018-199615060-00006. Drug Saf. 1996. PMID: 8968696 Review.
-
Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen.Cochrane Database Syst Rev. 2020 Dec 21;12(12):CD007245. doi: 10.1002/14651858.CD007245.pub4. Cochrane Database Syst Rev. 2020. PMID: 33348436 Free PMC article.
Cited by
-
Women's experience, satisfaction, and continuation with the levonogestrel-containing intrauterine system: A cross-sectional study.Medicine (Baltimore). 2024 Dec 27;103(52):e41063. doi: 10.1097/MD.0000000000041063. Medicine (Baltimore). 2024. PMID: 39969376 Free PMC article.
-
Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause.Fertil Steril. 2016 Dec;106(7):1588-1599. doi: 10.1016/j.fertnstert.2016.09.046. Fertil Steril. 2016. PMID: 27912889 Free PMC article. Review.
-
Progestin Intrauterine Devices and Metformin: Endometrial Hyperplasia and Early Stage Endometrial Cancer Medical Management.Healthcare (Basel). 2017 Jul 8;5(3):30. doi: 10.3390/healthcare5030030. Healthcare (Basel). 2017. PMID: 28698465 Free PMC article.
-
Considerations on a new, frameless copper-releasing intrauterine system for intracesarean insertion and its future clinical significance: A review.J Turk Ger Gynecol Assoc. 2020 Jun 8;21(2):130-133. doi: 10.4274/jtgga.galenos.2020.2020.0003. J Turk Ger Gynecol Assoc. 2020. PMID: 32517439 Free PMC article.
References
-
- French RS, Guillebaud J. Mirena®—the levonorgestrel intrauterine system (20 mcg/day) J Drug Delivery. 2003;1:43–70.
-
- Nillson CG, Johansson EDB, Luukkainen TA. A δ-norgestrel releasing IUD. Contraception. 1975;13:503–514. - PubMed
-
- Sivin I, Shaaban M, Odlind V, et al. A randomized trial of the Gyne T 380 and Gyne T 380 slimline intrauterine copper devices. Contraception. 1990;42:379–389. - PubMed
-
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova-T) IUD during five years of use: a randomised comparative trial. Contraception. 1994;49:56–72. - PubMed
-
- Indian Council of Medical Research Task Force on IUDs Randomized clinical trial with intrauterine devices (levonorgestrel intrauterine device (LNG), Cu T 380Ag, Cu T 220C and Cu T 200B). A 36-month study. Contraception. 1989;39:37–52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

