Recent advances in cohesin biology
- PMID: 27547382
- PMCID: PMC4975370
- DOI: 10.12688/f1000research.8881.1
Recent advances in cohesin biology
Abstract
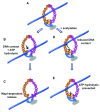
Sister chromatids are tethered together from the time they are formed in S-phase until they separate at anaphase. A protein complex called cohesin is responsible for holding the sister chromatids together and serves important roles in chromosome condensation, gene regulation, and the repair of DNA damage. Cohesin contains an open central pore and becomes topologically engaged with its DNA substrates. Entrapped DNA can be released either by the opening of a gate in the cohesin ring or by proteolytic cleavage of a component of the ring. This review summarizes recent research that provides important new insights into how DNA enters and exits the cohesin ring and how the rings behave on entrapped DNA molecules to provide functional cohesion.
Keywords: chromosome; cohesin; mitosis.
Conflict of interest statement
No competing interests were disclosed.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

