Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks
- PMID: 27547567
- PMCID: PMC4975003
- DOI: 10.7717/peerj.2261
Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks
Abstract
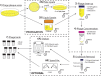
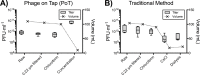
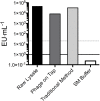
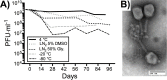
A major limitation with traditional phage preparations is the variability in titer, salts, and bacterial contaminants between successive propagations. Here we introduce the Phage On Tap (PoT) protocol for the quick and efficient preparation of homogenous bacteriophage (phage) stocks. This method produces homogenous, laboratory-scale, high titer (up to 10(10-11) PFU·ml(-1)), endotoxin reduced phage banks that can be used to eliminate the variability between phage propagations and improve the molecular characterizations of phage. The method consists of five major parts, including phage propagation, phage clean up by 0.22 μm filtering and chloroform treatment, phage concentration by ultrafiltration, endotoxin removal, and the preparation and storage of phage banks for continuous laboratory use. From a starting liquid lysate of > 100 mL, the PoT protocol generated a clean, homogenous, laboratory phage bank with a phage recovery efficiency of 85% within just two days. In contrast, the traditional method took upwards of five days to produce a high titer, but lower volume phage stock with a recovery efficiency of only 4%. Phage banks can be further purified for the removal of bacterial endotoxins, reducing endotoxin concentrations by over 3,000-fold while maintaining phage titer. The PoT protocol focused on T-like phages, but is broadly applicable to a variety of phages that can be propagated to sufficient titer, producing homogenous, high titer phage banks that are applicable for molecular and cellular assays.
Keywords: Bacteriophage; Cesium chloride; Dialysis; Endotoxin; Phage bank; Speed vacuum; Top agar; Ultrafiltration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Ackermann H-W, Tremblay D, Moineau S. Long-term bacteriophage preservation. WFCC Newsletter. 2004;38:35–40.
-
- Adams MH. Bacteriophages. New York: Interscience; 1959.
-
- Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, Salamon P, Youle M, Rohwer F. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):10771–10776. doi: 10.1073/pnas.1305923110. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

