Epidermal skin grafting in vitiligo: a pilot study
- PMID: 27547963
- PMCID: PMC7950019
- DOI: 10.1111/iwj.12632
Epidermal skin grafting in vitiligo: a pilot study
Abstract
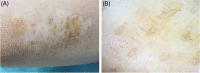
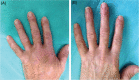
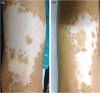
Vitiligo is a multifactorial acquired dermatosis characterised by achromic or hypochromic macules and by the absence of functioning melanocytes. Treatment depends on the extent of the affected areas and on disease activity. Surgical techniques have proven to be effective in stable cases but can be time-consuming and, in some cases, aesthetically unsatisfying or painful for the patients. The aim of the study was to assess the clinical safety and effectiveness of a new automatic epidermal skin harvesting device in patients with stable localised vitiligo over a minimum 12-month period. This new system (CELLUTOME™ Epidermal Harvesting System, KCI, an ACELITY Company, San Antonio, TX) is a commercially available epidermal skin harvesting system that can be used without local anaesthesia or other pre-treatments and has been shown to have low rates of donor site morbidity. Epidermal skin grafts can used in patients with acute and hard to heal chronic wounds, burns and stable vitiligo. The use of advanced therapies may improve the quality of life, have cost benefits and accelerate re-pigmentation of patients with vitiligo. In our preliminary study, this system was seen to be a safe and efficacious means of harvesting epidermal micrografts containing melanocytes for use in patients with stable vitiligo unresponsive to standard therapies.
Keywords: Epidermal skin grafting; Skin grafting; Vitiligo.
© 2016 Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures

Similar articles
-
Re: Epidermal skin grafting in vitiligo: a pilot study.Int Wound J. 2017 Dec;14(6):1401. doi: 10.1111/iwj.12774. Epub 2017 Jun 23. Int Wound J. 2017. PMID: 28643421 Free PMC article. No abstract available.
-
Epidermal curettage technique (ECT) for tissue harvest from the donor area for melanocyte autologous grafting in cases of vitiligo.An Bras Dermatol. 2014 Jul-Aug;89(4):681-3. doi: 10.1590/abd1806-4841.20142865. An Bras Dermatol. 2014. PMID: 25054767 Free PMC article.
-
Treatment of stable vitiligo hands by ReCell system: a preliminary report.Eur Rev Med Pharmacol Sci. 2010 Aug;14(8):691-4. Eur Rev Med Pharmacol Sci. 2010. PMID: 20707289
-
Use of epidermal grafts in wounds: a review of an automated epidermal harvesting system.J Wound Care. 2015 Apr;24(4 Suppl):30-4. doi: 10.12968/jowc.2015.24.Sup4b.30. J Wound Care. 2015. PMID: 25853646 Review.
-
Management of vitiligo patients with surgical interventions.Cutis. 2016 May;97(5):E27-9. Cutis. 2016. PMID: 27274556 Review.
Cited by
-
Management of the refractory vitiligo patient: current therapeutic strategies and future options.Front Immunol. 2024 Jan 4;14:1294919. doi: 10.3389/fimmu.2023.1294919. eCollection 2023. Front Immunol. 2024. PMID: 38239366 Free PMC article. Review.
-
Surgical Treatment of Vitiligo.Int J Environ Res Public Health. 2022 Apr 15;19(8):4812. doi: 10.3390/ijerph19084812. Int J Environ Res Public Health. 2022. PMID: 35457678 Free PMC article. Review.
-
Monoclonal Antibodies in the Management of Inflammation in Wound Healing: An Updated Literature Review.J Clin Med. 2024 Jul 12;13(14):4089. doi: 10.3390/jcm13144089. J Clin Med. 2024. PMID: 39064129 Free PMC article. Review.
-
Systematic review and meta-analysis of the use of micrografting technology in humans.J Int Med Res. 2025 May;53(5):3000605251337859. doi: 10.1177/03000605251337859. Epub 2025 May 24. J Int Med Res. 2025. PMID: 40411816 Free PMC article.
-
Wood's lamp for vitiligo disease stability and early recognition of initiative pigmentation after epidermal grafting.Int Wound J. 2017 Dec;14(6):1391-1394. doi: 10.1111/iwj.12800. Epub 2017 Aug 10. Int Wound J. 2017. PMID: 28799192 Free PMC article.
References
-
- Meurer M, Schild M. Treatment of vitiligo. Hautarzt 2016;67:249–64. - PubMed
-
- Boschi E, Longoni BM, Romanelli M, Mosca F. Cutaneous tissue engineering and lower extremity wounds (part 1). Int J Low Extrem Wounds 2004;3:80–6. - PubMed
-
- Cervelli V, Spallone D, Lucarini L, Palla L, Brinci L, De Angelis B. Treatment of stable vitiligo hands by ReCell system: a preliminary report. Eur Rev Med Pharmacol Sci 2010;14:691–4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

