Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc
- PMID: 27547982
- PMCID: PMC5080634
- DOI: 10.1021/acschembio.6b00453
Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc
Abstract
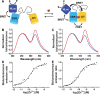
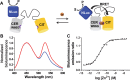
Genetically encoded FRET-based sensor proteins have significantly contributed to our current understanding of the intracellular functions of Zn2+. However, the external excitation required for these fluorescent sensors can give rise to photobleaching and phototoxicity during long-term imaging, limits applications that suffer from autofluorescence and light scattering, and is not compatible with light-sensitive cells. For these applications, sensor proteins based on Bioluminescence Resonance Energy Transfer (BRET) would provide an attractive alternative. In this work, we used the bright and stable luciferase NanoLuc to create the first genetically encoded BRET sensors for measuring intracellular Zn2+. Using a new sensor approach, the NanoLuc domain was fused to the Cerulean donor domain of two previously developed FRET sensors, eCALWY and eZinCh-2. In addition to preserving the excellent Zn2+ affinity and specificity of their predecessors, these newly developed sensors enable both BRET- and FRET-based detection. While the dynamic range of the BRET signal for the eCALWY-based BLCALWY-1 sensor was limited by the presence of two competing BRET pathways, BRET/FRET sensors based on the eZinCh-2 scaffold (BLZinCh-1 and -2) yielded robust 25-30% changes in BRET ratio. In addition, introduction of a chromophore-silencing mutation resulted in a BRET-only sensor (BLZinCh-3) with increased BRET response (50%) and an unexpected 10-fold increase in Zn2+ affinity. The combination of robust ratiometric response, physiologically relevant Zn2+ affinities, and stable and bright luminescence signal offered by the BLZinCh sensors allowed monitoring of intracellular Zn2+ in plate-based assays as well as intracellular BRET-based imaging in single living cells in real time.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Vallee B. L.; Falchuk K. H. (1993) The biochemical basis of zinc physiology. Physiol Rev. 73, 79–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

