Order, Disorder, and Everything in Between
- PMID: 27548131
- PMCID: PMC6274243
- DOI: 10.3390/molecules21081090
Order, Disorder, and Everything in Between
Abstract
In addition to the "traditional" proteins characterized by the unique crystal-like structures needed for unique functions, it is increasingly recognized that many proteins or protein regions (collectively known as intrinsically disordered proteins (IDPs) and intrinsically disordered protein regions (IDPRs)), being biologically active, do not have a specific 3D-structure in their unbound states under physiological conditions. There are also subtler categories of disorder, such as conditional (or dormant) disorder and partial disorder. Both the ability of a protein/region to fold into a well-ordered functional unit or to stay intrinsically disordered but functional are encoded in the amino acid sequence. Structurally, IDPs/IDPRs are characterized by high spatiotemporal heterogeneity and exist as dynamic structural ensembles. It is important to remember, however, that although structure and disorder are often treated as binary states, they actually sit on a structural continuum.
Keywords: flexible; intrinsically disordered; multi-functionality; protein function; structural heterogeneity; unfolded; unstructured.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
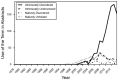





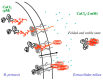
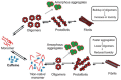



Similar articles
-
Proteins without unique 3D structures: biotechnological applications of intrinsically unstable/disordered proteins.Biotechnol J. 2015 Mar;10(3):356-66. doi: 10.1002/biot.201400374. Epub 2014 Oct 6. Biotechnol J. 2015. PMID: 25287424 Review.
-
Functions of short lifetime biological structures at large: the case of intrinsically disordered proteins.Brief Funct Genomics. 2020 Jan 22;19(1):60-68. doi: 10.1093/bfgp/ely023. Brief Funct Genomics. 2020. PMID: 29982297 Review.
-
Intrinsically disordered proteins in crowded milieu: when chaos prevails within the cellular gumbo.Cell Mol Life Sci. 2018 Nov;75(21):3907-3929. doi: 10.1007/s00018-018-2894-9. Epub 2018 Jul 31. Cell Mol Life Sci. 2018. PMID: 30066087 Free PMC article. Review.
-
Intrinsically disordered proteins and structured proteins with intrinsically disordered regions have different functional roles in the cell.PLoS One. 2019 Aug 19;14(8):e0217889. doi: 10.1371/journal.pone.0217889. eCollection 2019. PLoS One. 2019. PMID: 31425549 Free PMC article.
-
Intrinsic Disorder, Protein-Protein Interactions, and Disease.Adv Protein Chem Struct Biol. 2018;110:85-121. doi: 10.1016/bs.apcsb.2017.06.005. Epub 2017 Jul 24. Adv Protein Chem Struct Biol. 2018. PMID: 29413001 Review.
Cited by
-
Experimental characterization of de novo proteins and their unevolved random-sequence counterparts.Nat Ecol Evol. 2023 Apr;7(4):570-580. doi: 10.1038/s41559-023-02010-2. Epub 2023 Apr 6. Nat Ecol Evol. 2023. PMID: 37024625 Free PMC article.
-
'Intelligent' proteins.Cell Mol Life Sci. 2025 Jun 14;82(1):239. doi: 10.1007/s00018-025-05770-1. Cell Mol Life Sci. 2025. PMID: 40515853 Free PMC article. Review.
-
Reorientational Dynamics of Amyloid-β from NMR Spin Relaxation and Molecular Simulation.J Phys Chem Lett. 2019 Jun 20;10(12):3369-3375. doi: 10.1021/acs.jpclett.9b01050. Epub 2019 Jun 6. J Phys Chem Lett. 2019. PMID: 31181936 Free PMC article.
-
Protein structure-function continuum model: Emerging nexuses between specificity, evolution, and structure.Protein Sci. 2024 Apr;33(4):e4968. doi: 10.1002/pro.4968. Protein Sci. 2024. PMID: 38532700 Free PMC article. Review.
-
Rapid prediction and analysis of protein intrinsic disorder.Protein Sci. 2022 Dec;31(12):e4496. doi: 10.1002/pro.4496. Protein Sci. 2022. PMID: 36334049 Free PMC article.
References
-
- Tanford C., Reynolds J. Nature’s Robots, a History of Proteins. Oxford University Press; Oxford, UK: New York, NY, USA: 2001.
-
- Jorpes J.E. Jacob Berzelius, his Life and Work (Trans. B. Steele) Almqvist & Wiksell; Stockholm, Sweden: 1970.
-
- Anfinsen C.B., Redfield R.R., Choate W.L., Page J., Carroll W.R. Studies on the gross structure, cross-linkages, and terminal sequences in ribonuclease. J. Biol. Chem. 1954;207:201–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

