Sex-Based Selectivity of PPARγ Regulation in Th1, Th2, and Th17 Differentiation
- PMID: 27548145
- PMCID: PMC5000743
- DOI: 10.3390/ijms17081347
Sex-Based Selectivity of PPARγ Regulation in Th1, Th2, and Th17 Differentiation
Abstract
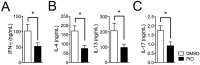
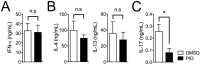
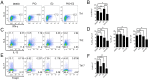
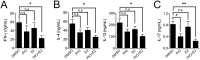
Peroxisome proliferator-activated receptor gamma (PPARγ) has recently been recognized to regulate adaptive immunity through Th17 differentiation, Treg functions, and TFH responses. However, its role in adaptive immunity and autoimmune disease is still not clear, possibly due to sexual differences. Here, we investigated in vitro treatment study with the PPARγ agonist pioglitazone to compare Th1, Th2, and Th17 differentiation in male and female mouse splenic T cells. Pioglitazone treatment significantly inhibited various effector T cell differentiations including Th1, Th2, and Th17 cells from female naïve T cells, but it selectively reduced IL-17 production in male Th17 differentiation. Interestingly, pioglitazone and estradiol (E2) co-treatment of T cells in males inhibited differentiation of Th1, Th2, and Th17 cells, suggesting a mechanism for the greater sensitivity of PPARγ to ligand treatment in the regulation of effector T cell differentiation in females. Collectively, these results demonstrate that PPARγ selectively inhibits Th17 differentiation only in male T cells and modulates Th1, Th2, and Th17 differentiation in female T cells based on different level of estrogen exposure. Accordingly, PPARγ could be an important immune regulator of sexual differences in adaptive immunity.
Keywords: PPARγ; effector T cells; estrogen; pioglitazone; sex.
Figures


Similar articles
-
Gender-specific differences in PPARγ regulation of follicular helper T cell responses with estrogen.Sci Rep. 2016 Jun 23;6:28495. doi: 10.1038/srep28495. Sci Rep. 2016. PMID: 27335315 Free PMC article.
-
Pioglitazone, a peroxisome proliferator-activated receptor gamma activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance.J Mol Cell Cardiol. 2005 Feb;38(2):257-65. doi: 10.1016/j.yjmcc.2004.11.010. Epub 2005 Jan 20. J Mol Cell Cardiol. 2005. PMID: 15698832
-
Adiponectin Suppresses T Helper 17 Cell Differentiation and Limits Autoimmune CNS Inflammation via the SIRT1/PPARγ/RORγt Pathway.Mol Neurobiol. 2017 Sep;54(7):4908-4920. doi: 10.1007/s12035-016-0036-7. Epub 2016 Aug 11. Mol Neurobiol. 2017. PMID: 27514756
-
PGI2 as a regulator of CD4+ subset differentiation and function.Prostaglandins Other Lipid Mediat. 2011 Nov;96(1-4):21-6. doi: 10.1016/j.prostaglandins.2011.08.003. Epub 2011 Aug 12. Prostaglandins Other Lipid Mediat. 2011. PMID: 21864703 Free PMC article. Review.
-
CD4(+) T-cell subsets in transplantation.Immunol Rev. 2013 Mar;252(1):183-91. doi: 10.1111/imr.12038. Immunol Rev. 2013. PMID: 23405905 Review.
Cited by
-
The role of GLS1-mediated glutaminolysis/2-HG/H3K4me3 and GSH/ROS signals in Th17 responses counteracted by PPARγ agonists.Theranostics. 2021 Mar 4;11(9):4531-4548. doi: 10.7150/thno.54803. eCollection 2021. Theranostics. 2021. PMID: 33754076 Free PMC article.
-
Significant Unresolved Questions and Opportunities for Bioengineering in Understanding and Treating COVID-19 Disease Progression.Cell Mol Bioeng. 2020 Jul 27;13(4):259-284. doi: 10.1007/s12195-020-00637-w. eCollection 2020 Aug. Cell Mol Bioeng. 2020. PMID: 32837585 Free PMC article.
-
PFAS and Potential Adverse Effects on Bone and Adipose Tissue Through Interactions With PPARγ.Endocrinology. 2021 Dec 1;162(12):bqab194. doi: 10.1210/endocr/bqab194. Endocrinology. 2021. PMID: 34480479 Free PMC article. Review.
-
Sex Hormones in Acquired Immunity and Autoimmune Disease.Front Immunol. 2018 Oct 4;9:2279. doi: 10.3389/fimmu.2018.02279. eCollection 2018. Front Immunol. 2018. PMID: 30337927 Free PMC article. Review.
-
Supraphysiological estradiol promotes human T follicular helper cell differentiation and favours humoural immunity during in vitro fertilization.J Cell Mol Med. 2021 Jul;25(14):6524-6534. doi: 10.1111/jcmm.16651. Epub 2021 May 24. J Cell Mol Med. 2021. PMID: 34032001 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

