Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals
- PMID: 27548152
- PMCID: PMC5000591
- DOI: 10.3390/ijms17081193
Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals
Abstract
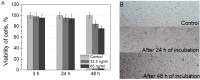
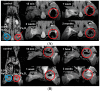
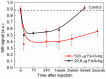
The uptake and distribution of negatively charged superparamagnetic iron oxide (Fe₃O₄) nanoparticles (SPIONs) in mouse embryonic fibroblasts NIH3T3, and magnetic resonance imaging (MRI) signal influenced by SPIONs injected into experimental animals, were visualized and investigated. Cellular uptake and distribution of the SPIONs in NIH3T3 after staining with Prussian Blue were investigated by a bright-field microscope equipped with digital color camera. SPIONs were localized in vesicles, mostly placed near the nucleus. Toxicity of SPION nanoparticles tested with cell viability assay (XTT) was estimated. The viability of NIH3T3 cells remains approximately 95% within 3-24 h of incubation, and only a slight decrease of viability was observed after 48 h of incubation. MRI studies on Wistar rats using a clinical 1.5 T MRI scanner were showing that SPIONs give a negative contrast in the MRI. The dynamic MRI measurements of the SPION clearance from the injection site shows that SPIONs slowly disappear from injection sites and only a low concentration of nanoparticles was completely eliminated within three weeks. No functionalized SPIONs accumulate in cells by endocytic mechanism, none accumulate in the nucleus, and none are toxic at a desirable concentration. Therefore, they could be used as a dual imaging agent: as contrast agents for MRI and for traditional optical biopsy by using Prussian Blue staining.
Keywords: MRI-optical dual imaging; SPIONs; cellular uptake; iron oxide; magnetic nanoparticles; multifunctional cancer diagnostics; optical biopsy of tissues cells.
Figures



Similar articles
-
One-pot facile synthesis of PEGylated superparamagnetic iron oxide nanoparticles for MRI contrast enhancement.Mater Sci Eng C Mater Biol Appl. 2014 Aug 1;41:161-7. doi: 10.1016/j.msec.2014.04.041. Epub 2014 Apr 28. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24907749
-
Efficient MRI labeling of endothelial progenitor cells: design of thiolated surface stabilized superparamagnetic iron oxide nanoparticles.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):346-55. doi: 10.1016/j.ejpb.2013.02.010. Epub 2013 Mar 7. Eur J Pharm Biopharm. 2013. PMID: 23481176
-
99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes.J Nucl Med. 2012 Mar;53(3):459-63. doi: 10.2967/jnumed.111.092437. Epub 2012 Feb 9. J Nucl Med. 2012. PMID: 22323777
-
Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation.AJR Am J Roentgenol. 2015 Mar;204(3):W302-13. doi: 10.2214/AJR.14.12733. AJR Am J Roentgenol. 2015. PMID: 25714316 Free PMC article. Review.
-
Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging.Contrast Media Mol Imaging. 2015 Sep-Oct;10(5):329-55. doi: 10.1002/cmmi.1638. Epub 2015 Apr 16. Contrast Media Mol Imaging. 2015. PMID: 25882768 Review.
Cited by
-
In Vitro Carcinoma Treatment Using Magnetic Nanocarriers under Ultrasound and Magnetic Fields.ACS Omega. 2018 May 31;3(5):5459-5469. doi: 10.1021/acsomega.8b00105. Epub 2018 May 21. ACS Omega. 2018. PMID: 30023921 Free PMC article.
-
A Novel Metal-Based Imaging Probe for Targeted Dual-Modality SPECT/MR Imaging of Angiogenesis.Front Chem. 2018 Jun 20;6:224. doi: 10.3389/fchem.2018.00224. eCollection 2018. Front Chem. 2018. PMID: 29974048 Free PMC article.
-
Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts.Sci Rep. 2018 Feb 27;8(1):3682. doi: 10.1038/s41598-018-22018-0. Sci Rep. 2018. PMID: 29487326 Free PMC article.
-
The effects of grafted mesenchymal stem cells labeled with iron oxide or cobalt-zinc-iron nanoparticles on the biological macromolecules of rat brain tissue extracts.Int J Nanomedicine. 2017 Jun 20;12:4519-4526. doi: 10.2147/IJN.S133156. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28684912 Free PMC article.
-
Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation.BMC Neurosci. 2017 Jun 26;18(1):51. doi: 10.1186/s12868-017-0369-9. BMC Neurosci. 2017. PMID: 28651647 Free PMC article. Review.
References
-
- Mitchell D.G., Cohen M.S., Saunders W.B. MRI Principles. Saunders; Philadelphia, PA, USA: 2004. p. 416.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

