Synthesis and Cytotoxicity against K562 Cells of 3-O-Angeloyl-20-O-acetyl Ingenol, a Derivative of Ingenol Mebutate
- PMID: 27548156
- PMCID: PMC5000744
- DOI: 10.3390/ijms17081348
Synthesis and Cytotoxicity against K562 Cells of 3-O-Angeloyl-20-O-acetyl Ingenol, a Derivative of Ingenol Mebutate
Abstract
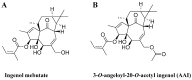
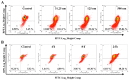
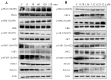
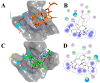
Ingenol mebutate possesses significant cytotoxicity and is clinically used to treat actinic keratosis. However, ingenol mebutate undergoes acyl migration which affects its bioactivity. Compound 3-O-angeloyl-20-O-acetyl ingenol (AAI, also known as 20-O-acetyl-ingenol-3-angelate or PEP008) is a synthetic derivative of ingenol mebutate. In this work, we report the AAI synthesis details and demonstrate AAI has higher cytotoxicity than ingenol mebutate in a chronic myeloid leukemia K562 cell line. Our data indicate that the increased activity of AAI originates from the improved intracellular stability of AAI rather than the increased binding affinity between AAI and the target protein protein kinase Cδ (PKCδ). AAI inhibits cell proliferation, induces G2/M phase arrest, disrupts the mitochondrial membrane potential, and stimulates apoptosis, as well as necrosis in K562 cells. Similar to ingenol mebutate, AAI activates PKCδ and extracellular signal regulated kinase (ERK), and inactivates protein kinase B (AKT). Furthermore, AAI also inhibits JAK/STAT3 pathway. Altogether, our studies show that ingenol derivative AAI is cytotoxic to K562 cells and modulates PKCδ/ERK, JAK/STAT3, and AKT signaling pathways. Our work suggests that AAI may be a new candidate of chemotherapeutic agent.
Keywords: 20-O-acetyl-ingenol-3-angelate; 3-O-angeloyl-20-O-acetyl ingenol; PEP008; apoptosis; chronic myeloid leukemia; ingenol mebutat.
Figures


Similar articles
-
Ingenol Mebutate Signals via PKC/MEK/ERK in Keratinocytes and Induces Interleukin Decoy Receptors IL1R2 and IL13RA2.Mol Cancer Ther. 2015 Sep;14(9):2132-42. doi: 10.1158/1535-7163.MCT-15-0023-T. Epub 2015 Jun 26. Mol Cancer Ther. 2015. PMID: 26116359
-
13-Oxyingenol dodecanoate, a cytotoxic ingenol derivative, induces mitochondrial apoptosis and caspase-dependent Akt decrease in K562 cells.Tumour Biol. 2016 May;37(5):6227-38. doi: 10.1007/s13277-015-4495-7. Epub 2015 Nov 28. Tumour Biol. 2016. PMID: 26615422
-
Synthesis, biological evaluation and SAR of 3-benzoates of ingenol for treatment of actinic keratosis and non-melanoma skin cancer.Bioorg Med Chem Lett. 2014 Jan 1;24(1):54-60. doi: 10.1016/j.bmcl.2013.11.073. Epub 2013 Dec 4. Bioorg Med Chem Lett. 2014. PMID: 24332494
-
Topical Ingenol Mebutate: A New Treatment Modality for Multiple Actinic Keratoses and Field Cancerization.Anticancer Agents Med Chem. 2017;17(10):1304-1311. doi: 10.2174/1871520617666170213130523. Anticancer Agents Med Chem. 2017. PMID: 28270072 Review.
-
Ingenane Diterpenoids.Prog Chem Org Nat Prod. 2016;102:1-90. doi: 10.1007/978-3-319-33172-0_1. Prog Chem Org Nat Prod. 2016. PMID: 27380406 Review.
References
-
- Tzogani K., Nagercoil N., Hemmings R.J., Samir B., Gardette J., Demolis P., Salmonson T., Pignatti F. The European Medicines Agency approval of ingenol mebutate (Picato) for the cutaneous treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis in adults: Summary of the scientific assessment of the Committee for Medicinal Products for Human Use (CHMP) Eur. J. Dermatol. 2014;24:457–463. - PubMed
-
- Hampson P., Wang K., Milverton L., Ersvaer E., Bruserud O., Lord J.M. Kinetics of ERK1/2 activation determine sensitivity of acute myeloid leukaemia cells to the induction of apoptosis by the novel small molecule ingenol 3-angelate (PEP005) Apoptosis. 2010;15:946–955. doi: 10.1007/s10495-010-0507-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

