A Cytochrome P450 3A4 Biosensor Based on Generation 4.0 PAMAM Dendrimers for the Detection of Caffeine
- PMID: 27548239
- PMCID: PMC5039663
- DOI: 10.3390/bios6030044
A Cytochrome P450 3A4 Biosensor Based on Generation 4.0 PAMAM Dendrimers for the Detection of Caffeine
Abstract
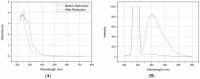
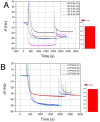
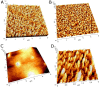
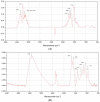
Cytochromes P450 (CYP, P450) are a large family of heme-active-site proteins involved in many catalytic processes, including steroidogenesis. In humans, four primary enzymes are involved in the metabolism of almost all xenobiotics. Among these enzymes, CYP3A4 is responsible for the inactivation of the majority of used drugs which makes this enzyme an interesting target for many fields of research, especially pharmaceutical research. Since the late 1970s, attempts have been made to construct and develop electrochemical sensors for the determination of substrates. This paper is concerned with the establishment of such a CYP3A4-containing biosensor. The sensor was constructed by adsorption of alternating layers of sub-nanometer gold particle-modified PAMAM (poly-amido-amine) dendrimers of generation 4.0, along with the enzyme by a layer-by-layer assembly technique. Atomic force microscopy (AFM), quartz crystal microbalance (QCM), and Fourier-transformed infrared spectroscopy (FTIR) were employed to elucidate the sensor assembly. Additionally, the biosensor was tested by cyclic voltammetry using caffeine as a substrate.
Keywords: PAMAM dendrimers; biosensor; cytochrome P450; electrochemistry.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Assembly of myoglobin layer-by-layer films with poly(propyleneimine) dendrimer-stabilized gold nanoparticles and its application in electrochemical biosensing.Biosens Bioelectron. 2007 Oct 31;23(3):393-9. doi: 10.1016/j.bios.2007.04.018. Epub 2007 May 5. Biosens Bioelectron. 2007. PMID: 17561388
-
E-DNA sensor of Mycobacterium tuberculosis based on electrochemical assembly of nanomaterials (MWCNTs/PPy/PAMAM).Anal Chem. 2015 Sep 15;87(18):9257-64. doi: 10.1021/acs.analchem.5b01761. Epub 2015 Aug 27. Anal Chem. 2015. PMID: 26313137
-
Cytochrome P450 biosensors-a review.Biosens Bioelectron. 2005 Jun 15;20(12):2408-23. doi: 10.1016/j.bios.2004.11.023. Epub 2005 Jan 5. Biosens Bioelectron. 2005. PMID: 15854816 Review.
-
Detection of parathyroid hormone using an electrochemical impedance biosensor based on PAMAM dendrimers.Biotechnol Prog. 2015 May-Jun;31(3):815-22. doi: 10.1002/btpr.2060. Epub 2015 Feb 26. Biotechnol Prog. 2015. PMID: 25683333
-
Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications.Talanta. 2016 Feb 1;148:427-38. doi: 10.1016/j.talanta.2015.11.022. Epub 2015 Nov 10. Talanta. 2016. PMID: 26653469 Review.
Cited by
-
Recent Advances in Electrochemical Biosensors Based on Enzyme Inhibition for Clinical and Pharmaceutical Applications.Sensors (Basel). 2018 Jan 9;18(1):164. doi: 10.3390/s18010164. Sensors (Basel). 2018. PMID: 29315246 Free PMC article. Review.
-
Electrochemical approaches based on micro- and nanomaterials for diagnosing oxidative stress.Mikrochim Acta. 2023 Mar 6;190(4):117. doi: 10.1007/s00604-023-05681-7. Mikrochim Acta. 2023. PMID: 36879086 Review.
-
Development of a dendrimer PAMAM‑based gold biochip for rapid and sensitive detection of endogenous IFN‑γ and anti‑IFN‑γ IgG in patients with hemophagocytic lymphohistiocytosis.Mol Med Rep. 2020 Dec;22(6):5369-5377. doi: 10.3892/mmr.2020.11605. Epub 2020 Oct 16. Mol Med Rep. 2020. PMID: 33173980 Free PMC article.
-
Recent Advances in Electrochemical Sensors for Caffeine Determination.Sensors (Basel). 2022 Nov 25;22(23):9185. doi: 10.3390/s22239185. Sensors (Basel). 2022. PMID: 36501886 Free PMC article. Review.
-
Novel Cytochrome P450-3A4 Enzymatic Nanobiosensor for Lapatinib (a Breast Cancer Drug) Developed on a Poly(anilino-co-4-aminobenzoic Acid-Green-Synthesised Indium Nanoparticle) Platform.Biosensors (Basel). 2023 Sep 21;13(9):897. doi: 10.3390/bios13090897. Biosensors (Basel). 2023. PMID: 37754131 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

