Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis
- PMID: 27548304
- PMCID: PMC5095490
- DOI: 10.1111/imm.12662
Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis
Erratum in
-
Correction to "Atorvastatin Promotes the Expansion of Myeloid-Derived Suppressor Cells and Attenuates Murine Colitis".Immunology. 2025 Jul 10. doi: 10.1111/imm.70009. Online ahead of print. Immunology. 2025. PMID: 40639943 No abstract available.
Abstract
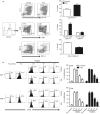
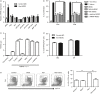
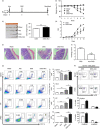
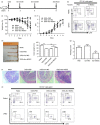
Statins, widely prescribed as cholesterol-lowering drugs, have recently been extensively studied for their pleiotropic effects on immune systems, especially their beneficial effects on autoimmune and inflammatory disorders. However, the mechanism of statin-induced immunosuppression is far from understood. Here, we found that atorvastatin promoted the expansion of myeloid-derived suppressor cells (MDSCs) both in vitro and in vivo. Atorvastatin-derived MDSCs suppressed T-cell responses by nitric oxide production. Addition of mevalonate, a downstream metabolite of 3-hydroxy-3-methylglutaryl coenzyme A reductase, almost completely abrogated the effect of atorvastatin on MDSCs, indicating that the mevalonate pathway was involved. Along with the amelioration of dextran sodium sulphate (DSS) -induced murine acute and chronic colitis, we observed a higher MDSC level both in spleen and intestine tissue compared with that from DSS control mice. More importantly, transfer of atorvastatin-derived MDSCs attenuated DSS acute colitis and T-cell transfer of chronic colitis. Hence, our data suggest that the expansion of MDSCs induced by statins may exert a beneficial effect on autoimmune diseases. In summary, our study provides a novel potential mechanism for statins-based treatment in inflammatory bowel disease and perhaps other autoimmune diseases.
Keywords: atorvastatin; immunosuppression; murine colitis; myeloid-derived suppressor cells; nitric oxide.
© 2016 John Wiley & Sons Ltd.
Figures


Similar articles
-
PMN-MDSCs are responsible for immune suppression in anti-PD-1 treated TAP1 defective melanoma.Clin Transl Oncol. 2025 Jul;27(7):3073-3083. doi: 10.1007/s12094-024-03840-7. Epub 2025 Jan 18. Clin Transl Oncol. 2025. PMID: 39825997
-
A derivative of tanshinone IIA and salviadione, 15a, inhibits inflammation and alleviates DSS-induced colitis in mice by direct binding and inhibition of RIPK2.Acta Pharmacol Sin. 2025 Mar;46(3):672-686. doi: 10.1038/s41401-024-01399-1. Epub 2024 Oct 23. Acta Pharmacol Sin. 2025. PMID: 39443729
-
Decitabine shows potent anti-myeloma activity by depleting monocytic myeloid-derived suppressor cells in the myeloma microenvironment.J Cancer Res Clin Oncol. 2019 Feb;145(2):329-336. doi: 10.1007/s00432-018-2790-6. Epub 2018 Nov 13. J Cancer Res Clin Oncol. 2019. PMID: 30426212 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.Health Technol Assess. 2007 Apr;11(14):1-160, iii-iv. doi: 10.3310/hta11140. Health Technol Assess. 2007. PMID: 17408535
Cited by
-
Treatment With Endothelin-A Receptor Antagonist BQ123 Attenuates Acute Inflammation in Mice Through T-Cell-Dependent Polymorphonuclear Myeloid-Derived Suppressor Cell Activation.Front Immunol. 2021 Mar 22;12:641874. doi: 10.3389/fimmu.2021.641874. eCollection 2021. Front Immunol. 2021. PMID: 33828553 Free PMC article.
-
Statins inhibit protein kinase D (PKD) activation in intestinal cells and prevent PKD1-induced growth of murine enteroids.Am J Physiol Cell Physiol. 2023 Apr 1;324(4):C807-C820. doi: 10.1152/ajpcell.00286.2022. Epub 2023 Feb 13. Am J Physiol Cell Physiol. 2023. PMID: 36779664 Free PMC article.
-
Statins, metformin, proprotein-convertase-subtilisin-kexin type-9 (PCSK9) inhibitors and sex hormones: Immunomodulatory properties?Rev Endocr Metab Disord. 2018 Dec;19(4):363-395. doi: 10.1007/s11154-018-9478-8. Rev Endocr Metab Disord. 2018. PMID: 30673921 Review.
-
Lipid Profiles in Patients With Ulcerative Colitis Receiving Tofacitinib-Implications for Cardiovascular Risk and Patient Management.Inflamm Bowel Dis. 2021 May 17;27(6):797-808. doi: 10.1093/ibd/izaa227. Inflamm Bowel Dis. 2021. PMID: 32870265 Free PMC article.
-
The paradoxical role of MDSCs in inflammatory bowel diseases: From bench to bedside.Front Immunol. 2022 Sep 15;13:1021634. doi: 10.3389/fimmu.2022.1021634. eCollection 2022. Front Immunol. 2022. PMID: 36189262 Free PMC article. Review.
References
-
- Ulivieri C, Baldari CT. Statins: from cholesterol‐lowering drugs to novel immunomodulators for the treatment of Th17‐mediated autoimmune diseases. Pharmacol Res 2014; 88:41–52. - PubMed
-
- Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, et al The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta‐analysis. Clin Exp Rheumatol 2015; 33:69–76. - PubMed
-
- Aktunc E, Kayhan B, Arasli M, Gun BD, Barut F. The effect of atorvastatin and its role on systemic cytokine network in treatment of acute experimental colitis. Immunopharmacol Immunotoxicol 2011; 33:667–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

