Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation
- PMID: 27548529
- PMCID: PMC5004955
- DOI: 10.1172/JCI84871
Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation
Abstract
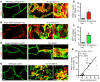
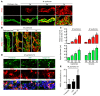
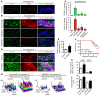
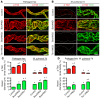
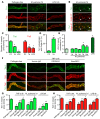
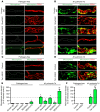
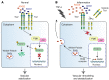
Angiopoietin-2 (ANG2) regulates blood vessel remodeling in many pathological conditions through differential effects on Tie2 signaling. While ANG2 competes with ANG1 to inhibit Tie2, it can paradoxically also promote Tie2 phosphorylation (p-Tie2). A related paradox is that both inactivation and overactivation of Tie2 can result in vascular remodeling. Here, we reconciled these opposing actions of ANG2 by manipulating conditions that govern its actions in the vasculature. ANG2 drove vascular remodeling during Mycoplasma pulmonis infection by acting as a Tie2 antagonist, which led to p-Tie2 suppression, forkhead box O1 (FOXO1) activation, increased ANG2 expression, and vessel leakiness. These changes were exaggerated by anti-Tie2 antibody, inhibition of PI3K signaling, or ANG2 overexpression and were reduced by anti-ANG2 antibody or exogenous ANG1. In contrast, under pathogen-free conditions, ANG2 drove vascular remodeling by acting as an agonist, promoting high p-Tie2, low FOXO1 activation, and no leakage. Tie1 activation was strong under pathogen-free conditions, but infection or TNF-α led to Tie1 inactivation by ectodomain cleavage and promoted the Tie2 antagonist action of ANG2. Together, these data indicate that ANG2 activation of Tie2 supports stable enlargement of normal nonleaky vessels, but reduction of Tie1 in inflammation leads to ANG2 antagonism of Tie2 and initiates a positive feedback loop wherein FOXO1-driven ANG2 expression promotes vascular remodeling and leakage.
Figures

Comment in
-
Tie1: an orphan receptor provides context for angiopoietin-2/Tie2 signaling.J Clin Invest. 2016 Sep 1;126(9):3188-91. doi: 10.1172/JCI89963. Epub 2016 Aug 22. J Clin Invest. 2016. PMID: 27548526 Free PMC article.
References
-
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

