Tumors Alter Inflammation and Impair Dermal Wound Healing in Female Mice
- PMID: 27548621
- PMCID: PMC4993492
- DOI: 10.1371/journal.pone.0161537
Tumors Alter Inflammation and Impair Dermal Wound Healing in Female Mice
Abstract
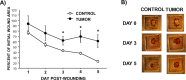
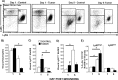
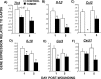
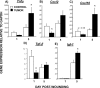
Tissue repair is an integral component of cancer treatment (e.g., due to surgery, chemotherapy, radiation). Previous work has emphasized the immunosuppressive effects of tumors on adaptive immunity and has shown that surgery incites cancer metastases. However, the extent to which and how tumors may alter the clinically-relevant innate immune process of wound healing remains an untapped potential area of improvement for treatment, quality of life, and ultimately, mortality of cancer patients. In this study, 3.5 mm full-thickness dermal excisional wounds were placed on the dorsum of immunocompetent female mice with and without non-malignant flank AT-84 murine oral squamous cell carcinomas. Wound closure rate, inflammatory cell number and inflammatory signaling in wounds, and circulating myeloid cell concentrations were compared between tumor-bearing and tumor-free mice. Tumors delayed wound closure, suppressed inflammatory signaling, and altered myeloid cell trafficking in wounds. An in vitro scratch "wounding" assay of adult dermal fibroblasts treated with tumor cell-conditioned media supported the in vivo findings. This study demonstrates that tumors are sufficient to disrupt fundamental and clinically-relevant innate immune functions. The understanding of these underlying mechanisms provides potential for therapeutic interventions capable of improving the treatment of cancer while reducing morbidities and mortality.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Effects of dermal wounding on distal primary tumor immunobiology in mice.J Surg Res. 2018 Jan;221:328-335. doi: 10.1016/j.jss.2017.09.016. Epub 2017 Oct 17. J Surg Res. 2018. PMID: 29229147 Free PMC article.
-
Partial thickness wound: Does mechanism of injury influence healing?Burns. 2019 May;45(3):531-542. doi: 10.1016/j.burns.2018.08.010. Epub 2019 Feb 8. Burns. 2019. PMID: 30739729
-
Loss of Atg7 in Endothelial Cells Enhanced Cutaneous Wound Healing in a Mouse Model.J Surg Res. 2020 May;249:145-155. doi: 10.1016/j.jss.2019.12.004. Epub 2020 Jan 17. J Surg Res. 2020. PMID: 31958599
-
The Bigger Picture: Why Oral Mucosa Heals Better Than Skin.Biomolecules. 2021 Aug 6;11(8):1165. doi: 10.3390/biom11081165. Biomolecules. 2021. PMID: 34439831 Free PMC article. Review.
-
The Cutaneous Wound Innate Immunological Microenvironment.Int J Mol Sci. 2020 Nov 19;21(22):8748. doi: 10.3390/ijms21228748. Int J Mol Sci. 2020. PMID: 33228152 Free PMC article. Review.
Cited by
-
Preoperative white blood cell count predicts anastomotic leakage in patients with left-sided colorectal cancer.PLoS One. 2021 Oct 20;16(10):e0258713. doi: 10.1371/journal.pone.0258713. eCollection 2021. PLoS One. 2021. PMID: 34669737 Free PMC article.
-
Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine.EPMA J. 2017 Mar 3;8(1):23-33. doi: 10.1007/s13167-017-0081-y. eCollection 2017 Mar. EPMA J. 2017. PMID: 28620441 Free PMC article. Review.
-
Euflammation Attenuates Central and Peripheral Inflammation and Cognitive Consequences of an Immune Challenge after Tumor Development.Neuroimmunomodulation. 2017;24(2):74-86. doi: 10.1159/000479184. Epub 2017 Sep 13. Neuroimmunomodulation. 2017. PMID: 28898868 Free PMC article.
-
Appraisal of cytotoxicity and acrylamide mitigation potential of L-asparaginase SlpA from fish gut microbiome.Appl Microbiol Biotechnol. 2022 May;106(9-10):3583-3598. doi: 10.1007/s00253-022-11954-7. Epub 2022 May 17. Appl Microbiol Biotechnol. 2022. PMID: 35579684
-
The composition of T-cell subsets are altered in the burn wound early after injury.PLoS One. 2017 Jun 2;12(6):e0179015. doi: 10.1371/journal.pone.0179015. eCollection 2017. PLoS One. 2017. PMID: 28575063 Free PMC article.
References
-
- de Araujo T, Valencia I, Federman DG, Kirsner RS. Managing the patient with venous ulcers. Ann Intern Med. 2003;138(4):326–34. Epub 2003/02/15. . - PubMed
-
- McNees P, Meneses KD. Pressure ulcers and other chronic wounds in patients with and patients without cancer: a retrospective, comparative analysis of healing patterns. Ostomy Wound Manage. 2007;53(2):70–8. Epub 2007/02/13. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

