Aberrant immune response with consequent vascular and connective tissue remodeling - causal to scleroderma and associated syndromes such as Raynaud phenomenon and other fibrosing syndromes?
- PMID: 27548652
- PMCID: PMC5114306
- DOI: 10.1097/BOR.0000000000000333
Aberrant immune response with consequent vascular and connective tissue remodeling - causal to scleroderma and associated syndromes such as Raynaud phenomenon and other fibrosing syndromes?
Abstract
Purpose of review: Scleroderma and other autoimmune-induced connective tissue diseases are characterized by dysfunctions in the immune system, connective tissue and the vasculature. We are focusing on systemic sclerosis (SSc)-associated pulmonary hypertension, which remains a leading cause of death with only a 50-60% of 2-year survival rate.
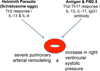
Recent findings: Much research and translational efforts have been directed at understanding the immune response that causes SSc and the networked interactions with the connective tissue and the vasculature. One of the unexpected findings was that in some cases the pathogenic immune response in SSc resembles the immune response to helminth parasites. During coevolution, means of communication were developed which protect the host from over-colonization with parasites and which protect the parasite from excessive host responses. One explanation for the geographically clustered occurrence of SSc is that environmental exposures combined with genetic predisposition turn on triggers of molecular and cellular modules that were once initiated by parasites.
Summary: Future research is needed to further understand the parasite-derived signals that dampen the host response. Therapeutic helminth infection or treatment with parasite-derived response modifiers could be promising new management tools for autoimmune connective tissue diseases.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA, Jr, Carreira PE, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755. - PubMed
-
- Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, Bancel DF, Allanore Y, Muller-Ladner U, Distler O, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. - PubMed
-
- Tan FK, Stivers DN, Arnett FC, Chakraborty R, Howard R, Reveille JD. HLA haplotypes and microsatellite polymorphisms in and around the major histocompatibility complex region in a Native American population with a high prevalence of scleroderma (systemic sclerosis) Tissue Antigens. 1999;53:74–80. - PubMed
-
- Arnett FC, Howard RF, Tan F, Moulds JM, Bias WB, Durban E, Cameron HD, Paxton G, Hodge TJ, Weathers PE, et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma. Association with an Amerindian HLA haplotype. Arthritis Rheum. 1996;39:1362–1370. - PubMed
-
-
Furukawa H, Oka S, Kawasaki A, Shimada K, Sugii S, Matsushita T, Hashimoto A, Komiya A, Fukui N, Kobayashi K, et al. Human Leukocyte Antigen and Systemic Sclerosis in Japanese: The Sign of the Four Independent Protective Alleles, DRB1*13:02, DRB1*14:06, DQB1*03:01, and DPB1*02:01. PLoS One. 2016;11:e0154255. beautiful example of molecular ethnic cluster of systemic sclerosis
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

