Transcription upregulation via force-induced direct stretching of chromatin
- PMID: 27548707
- PMCID: PMC5121013
- DOI: 10.1038/nmat4729
Transcription upregulation via force-induced direct stretching of chromatin
Abstract
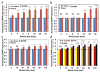
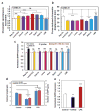
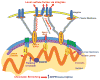
Mechanical forces play critical roles in the function of living cells. However, the underlying mechanisms of how forces influence nuclear events remain elusive. Here, we show that chromatin deformation as well as force-induced transcription of a green fluorescent protein (GFP)-tagged bacterial-chromosome dihydrofolate reductase (DHFR) transgene can be visualized in a living cell by using three-dimensional magnetic twisting cytometry to apply local stresses on the cell surface via an Arg-Gly-Asp-coated magnetic bead. Chromatin stretching depended on loading direction. DHFR transcription upregulation was sensitive to load direction and proportional to the magnitude of chromatin stretching. Disrupting filamentous actin or inhibiting actomyosin contraction abrogated or attenuated force-induced DHFR transcription, whereas activating endogenous contraction upregulated force-induced DHFR transcription. Our findings suggest that local stresses applied to integrins propagate from the tensed actin cytoskeleton to the LINC complex and then through lamina-chromatin interactions to directly stretch chromatin and upregulate transcription.
Conflict of interest statement
Conflict of Interest. The authors declare no conflict of interest.
Figures



Comment in
-
Cell mechanotransduction: Stretch to express.Nat Mater. 2016 Nov 23;15(12):1227-1229. doi: 10.1038/nmat4809. Nat Mater. 2016. PMID: 27876751 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

