Fiber Network Models Predict Enhanced Cell Mechanosensing on Fibrous Gels
- PMID: 27548709
- PMCID: PMC5018119
- DOI: 10.1115/1.4034490
Fiber Network Models Predict Enhanced Cell Mechanosensing on Fibrous Gels
Abstract
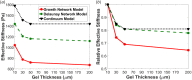
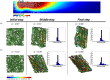
The propagation of mechanical signals through nonlinear fibrous tissues is much more extensive than through continuous synthetic hydrogels. Results from recent studies indicate that increased mechanical propagation arises from the fibrous nature of the material rather than the strain-stiffening property. The relative importance of different parameters of the fibrous network structure to this propagation, however, remains unclear. In this work, we directly compared the mechanical response of substrates of varying thickness subjected to a constant cell traction force using either a nonfibrous strain-stiffening continuum-based model or a volume-averaged fiber network model consisting of two different types of fiber network structures: one with low fiber connectivity (growth networks) and one with high fiber connectivity (Delaunay networks). The growth network fiber models predicted a greater propagation of substrate displacements through the model and a greater sensitivity to gel thickness compared to the more connected Delaunay networks and the nonlinear continuum model. Detailed analysis of the results indicates that rotational freedom of the fibers in a network with low fiber connectivity is critically important for enhanced, long-range mechanosensing. Our findings demonstrate the utility of multiscale models in predicting cells mechanosensing on fibrous gels, and they provide a more complete understanding of how cell traction forces propagate through fibrous tissues, which has implications for the design of engineered tissues and the stem cell niche.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

