Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis
- PMID: 27548819
- PMCID: PMC5546305
- DOI: 10.1097/CCM.0000000000002052
Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis
Abstract
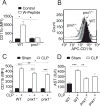
Objective: Sepsis remains an unresolved clinical problem. Therapeutic strategies focusing on inhibition of neutrophils (polymorphonuclear neutrophils) have failed, which indicates that a more detailed understanding of the underlying pathophysiology of sepsis is required. Polymorphonuclear neutrophil activation and chemotaxis require cellular adenosine triphosphate release via pannexin-1 channels that fuel autocrine feedback via purinergic receptors. In the current study, we examined the roles of endogenous and systemic adenosine triphosphate on polymorphonuclear neutrophil activation and host defense in sepsis.
Design: Prospective randomized animal investigation and in vitro studies.
Setting: Preclinical academic research laboratory.
Subjects: Wild-type C57BL/6 mice, pannexin-1 knockout mice, and healthy human subjects used to obtain polymorphonuclear neutrophils for in vitro studies.
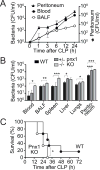
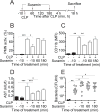
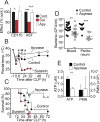
Interventions: Wild-type and pannexin-1 knockout mice were treated with suramin or apyrase to block the endogenous or systemic effects of adenosine triphosphate. Mice were subjected to cecal ligation and puncture and polymorphonuclear neutrophil activation (CD11b integrin expression), organ (liver) injury (plasma aspartate aminotransferase), bacterial spread, and survival were monitored. Human polymorphonuclear neutrophils were used to study the effect of systemic adenosine triphosphate and apyrase on chemotaxis.
Measurements and main results: Inhibiting endogenous adenosine triphosphate reduced polymorphonuclear neutrophil activation and organ injury, but increased the spread of bacteria and mortality in sepsis. By contrast, removal of systemic adenosine triphosphate improved bacterial clearance and survival in sepsis by improving polymorphonuclear neutrophil chemotaxis.
Conclusions: Systemic adenosine triphosphate impairs polymorphonuclear neutrophil functions by disrupting the endogenous purinergic signaling mechanisms that regulate cell activation and chemotaxis. Removal of systemic adenosine triphosphate improves polymorphonuclear neutrophil function and host defenses, making this a promising new treatment strategy for sepsis.
Figures
Similar articles
-
Frontline Science: Escherichia coli use LPS as decoy to impair neutrophil chemotaxis and defeat antimicrobial host defense.J Leukoc Biol. 2019 Dec;106(6):1211-1219. doi: 10.1002/JLB.4HI0319-109R. Epub 2019 Aug 8. J Leukoc Biol. 2019. PMID: 31392789 Free PMC article.
-
Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis.Crit Care Med. 2008 Sep;36(9):2569-75. doi: 10.1097/CCM.0b013e3181841a91. Crit Care Med. 2008. PMID: 18679117 Free PMC article.
-
Plasma ATP is required for neutrophil activation in a mouse sepsis model.Shock. 2014 Aug;42(2):142-7. doi: 10.1097/SHK.0000000000000180. Shock. 2014. PMID: 24675414 Free PMC article.
-
The role of neutrophils in severe sepsis.Shock. 2008 Oct;30 Suppl 1:3-9. doi: 10.1097/SHK.0b013e3181818466. Shock. 2008. PMID: 18704017 Review.
-
Purinergic regulation of neutrophil chemotaxis.Cell Mol Life Sci. 2008 Aug;65(16):2528-40. doi: 10.1007/s00018-008-8095-1. Cell Mol Life Sci. 2008. PMID: 18463789 Free PMC article. Review.
Cited by
-
Neutrophils: fast and furious-the nucleotide pathway.Purinergic Signal. 2021 Sep;17(3):371-383. doi: 10.1007/s11302-021-09786-7. Epub 2021 Apr 29. Purinergic Signal. 2021. PMID: 33913070 Free PMC article. Review.
-
Optimized HPLC method to elucidate the complex purinergic signaling dynamics that regulate ATP, ADP, AMP, and adenosine levels in human blood.Purinergic Signal. 2022 Jun;18(2):223-239. doi: 10.1007/s11302-022-09842-w. Epub 2022 Feb 7. Purinergic Signal. 2022. PMID: 35132577 Free PMC article.
-
Cardiomyocyte PANX1 Controls Glycolysis and Neutrophil Recruitment in Hypertrophy.Circ Res. 2024 Aug 2;135(4):503-517. doi: 10.1161/CIRCRESAHA.124.324650. Epub 2024 Jul 3. Circ Res. 2024. PMID: 38957990 Free PMC article.
-
Enhanced Macrophage Pannexin 1 Expression and Hemichannel Activation Exacerbates Lethal Experimental Sepsis.Sci Rep. 2019 Jan 17;9(1):160. doi: 10.1038/s41598-018-37232-z. Sci Rep. 2019. PMID: 30655582 Free PMC article.
-
Mitochondria Synergize With P2 Receptors to Regulate Human T Cell Function.Front Immunol. 2020 Sep 29;11:549889. doi: 10.3389/fimmu.2020.549889. eCollection 2020. Front Immunol. 2020. PMID: 33133068 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

