Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1
- PMID: 27549031
- PMCID: PMC4994088
- DOI: 10.1038/srep32084
Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1
Abstract
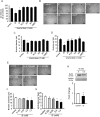
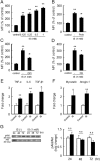
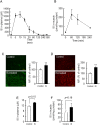
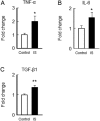
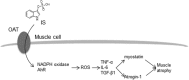
Skeletal muscle atrophy, referred to as sarcopenia, is often observed in chronic kidney disease (CKD) patients, especially in patients who are undergoing hemodialysis. The purpose of this study was to determine whether uremic toxins are involved in CKD-related skeletal muscle atrophy. Among six protein-bound uremic toxins, indole containing compounds, indoxyl sulfate (IS) significantly inhibited proliferation and myotube formation in C2C12 myoblast cells. IS increased the factors related to skeletal muscle breakdown, such as reactive oxygen species (ROS) and inflammatory cytokines (TNF-α, IL-6 and TGF-β1) in C2C12 cells. IS also enhanced the production of muscle atrophy-related genes, myostatin and atrogin-1. These effects induced by IS were suppressed in the presence of an antioxidant or inhibitors of the organic anion transporter and aryl hydrocarbon receptor. The administered IS was distributed to skeletal muscle and induced superoxide production in half-nephrectomized (1/2 Nx) mice. The chronic administration of IS significantly reduced the body weights accompanied by skeletal muscle weight loss. Similar to the in vitro data, IS induced the expression of myostatin and atrogin-1 in addition to increasing the production of inflammatory cytokines by enhancing oxidative stress in skeletal muscle. These data suggest that IS has the potential to accelerate skeletal muscle atrophy by inducing oxidative stress-mediated myostatin and atrogin-1 expression.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

