Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice
- PMID: 27549339
- PMCID: PMC4996648
- DOI: 10.7554/eLife.16351
Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice
Abstract
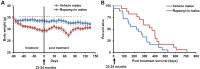
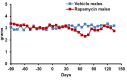
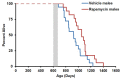
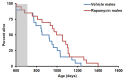
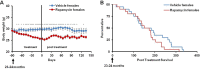
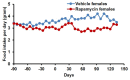
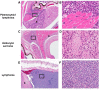
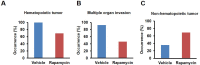
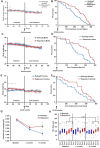
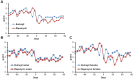
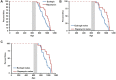
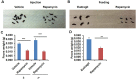
The FDA approved drug rapamycin increases lifespan in rodents and delays age-related dysfunction in rodents and humans. Nevertheless, important questions remain regarding the optimal dose, duration, and mechanisms of action in the context of healthy aging. Here we show that 3 months of rapamycin treatment is sufficient to increase life expectancy by up to 60% and improve measures of healthspan in middle-aged mice. This transient treatment is also associated with a remodeling of the microbiome, including dramatically increased prevalence of segmented filamentous bacteria in the small intestine. We also define a dose in female mice that does not extend lifespan, but is associated with a striking shift in cancer prevalence toward aggressive hematopoietic cancers and away from non-hematopoietic malignancies. These data suggest that a short-term rapamycin treatment late in life has persistent effects that can robustly delay aging, influence cancer prevalence, and modulate the microbiome.
Keywords: aging; cancer; cancer biology; developmental biology; healthspan; longevity; mTOR; microbiome; mouse; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
-
- Anderson MJ. A new method for non‐parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. - DOI
-
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nuñez G, Camp JG, Hattori M, Umesaki Y, Honda K, Yokoyama Shin-ichiro, Morita H. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. - DOI - PMC - PubMed
-
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. https://arxiv.org/abs/1406.5823 arXiv. 2014:e16351.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

