Mesothelioma response to carbon nanotubes is associated with an early and selective accumulation of immunosuppressive monocytic cells
- PMID: 27549627
- PMCID: PMC4994252
- DOI: 10.1186/s12989-016-0158-0
Mesothelioma response to carbon nanotubes is associated with an early and selective accumulation of immunosuppressive monocytic cells
Abstract
Background: The asbestos-like toxicity of some engineered carbon nanotubes (CNT), notably their capacity to induce mesothelioma, is a serious cause of concern for public health. Here we show that carcinogenic CNT induce an early and sustained immunosuppressive response characterized by the accumulation of monocytic Myeloid Derived Suppressor Cells (M-MDSC) that counteract effective immune surveillance of tumor cells.
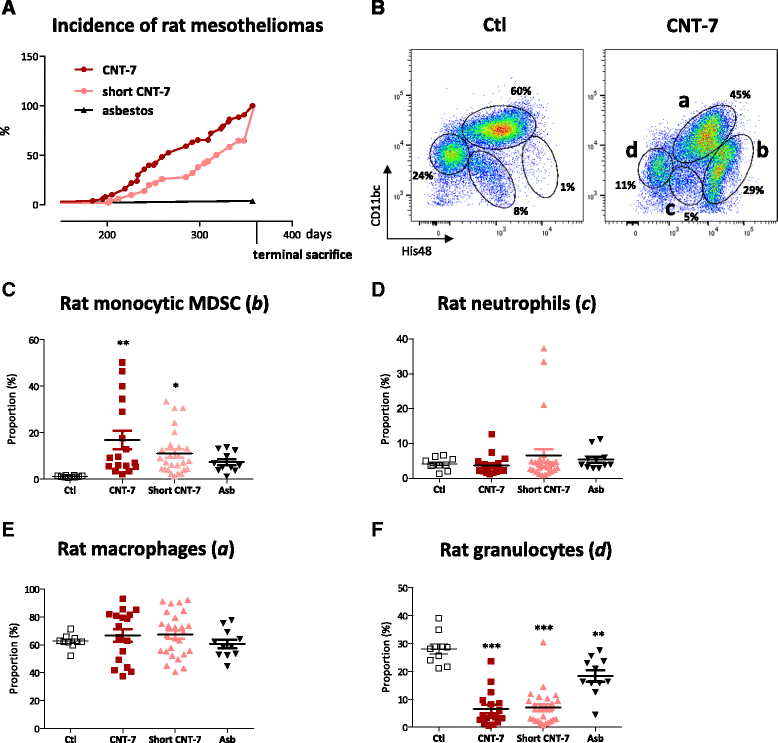
Methods: Wistar rats and C57BL/6 mice were intraperitoneally injected with carcinogenic multi-walled Mitsui-7 CNT (CNT-7) or crocidolite asbestos. Peritoneal mesothelioma development and immune cell accumulation were assessed until 12 months. Leukocyte sub-populations were identified by recording expression of CD11b/c and His48 by flow cytometry. The immunosuppressive activity on T lymphocytes of purified peritoneal leukocytes was assessed in a co-culture assay with activated spleen cells.
Results: We demonstrate that long and short mesotheliomagenic CNT-7 injected in the peritoneal cavity of rats induced, like asbestos, an early and selective accumulation of monocytic cells (CD11b/c(int) and His48(hi)) which possess the ability to suppress polyclonal activation of T lymphocytes and correspond to M-MDSC. Peritoneal M-MDSC persisted during the development of peritoneal mesothelioma in CNT-7-treated rats but were only transiently recruited after non-carcinogenic CNT (CNT-M, CNT-T) injection. Peritoneal M-MDSC did not accumulate in mice which are resistant to mesothelioma development.
Conclusions: Our data provide new insights into the initial pathogenic events induced by CNT, adding a new component to the adverse outcome pathway leading to mesothelioma development. The specificity of the M-MDSC response after carcinogenic CNT exposure highlights the interest of this response for detecting the ability of new nanomaterials to cause cancer.
Keywords: Asbestos; Carbon nanotubes; Crocidolite; Fibers; Immunosuppression; Inflammation; Mesothelioma; Myeloid cells; Rats and mice.
Figures


References
-
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014;15:1427–1428. doi: 10.1016/S1470-2045(14)71109-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

