Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence
- PMID: 27549662
- PMCID: PMC4994321
- DOI: 10.1186/s12936-016-1468-2
Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence
Abstract
Background: Malaria exerts a tremendous socioeconomic impact worldwide despite current control efforts, and novel disease transmission-blocking strategies are urgently needed. The Enterobacter bacterium Esp_Z, which is naturally harboured in the mosquito midgut, can inhibit the development of Plasmodium parasites prior to their invasion of the midgut epithelium through a mechanism that involves oxidative stress. Here, a multifaceted approach is used to study the tripartite interactions between the mosquito, Esp_Z and Plasmodium, towards addressing the feasibility of using sugar-baited exposure of mosquitoes to the Esp_Z bacterium for interruption of malaria transmission.
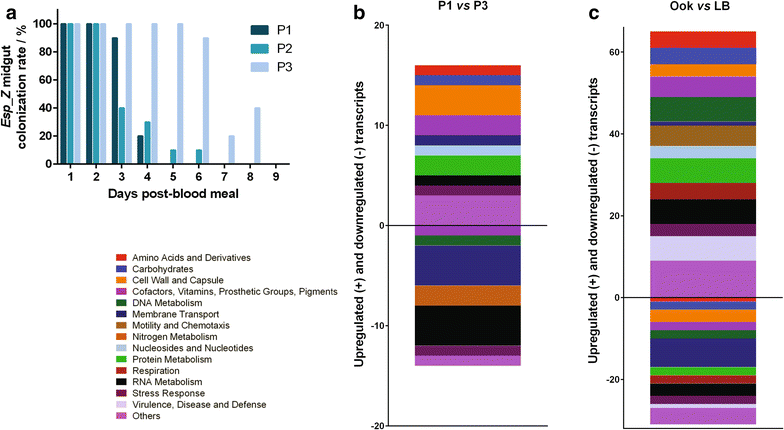
Methods: The ability of Esp_Z to colonize Anopheles gambiae midguts harbouring microbiota derived from wild mosquitoes was determined by qPCR. Upon introduction of Esp_Z via nectar feeding, the permissiveness of colonized mosquitoes to Plasmodium falciparum infection was determined, as well as the impact of Esp_Z on mosquito fitness parameters, such as longevity, number of eggs laid and number of larvae hatched. The genome of Esp_Z was sequenced, and transcriptome analyses were performed to identify bacterial genes that are important for colonization of the mosquito midgut, as well as for ROS-production. A gene expression analysis of members of the oxidative defence pathway of Plasmodium berghei was also conducted to assess the parasite's oxidative defence response to Esp_Z exposure.
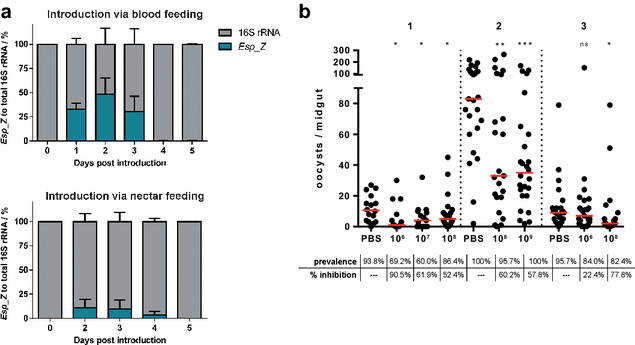
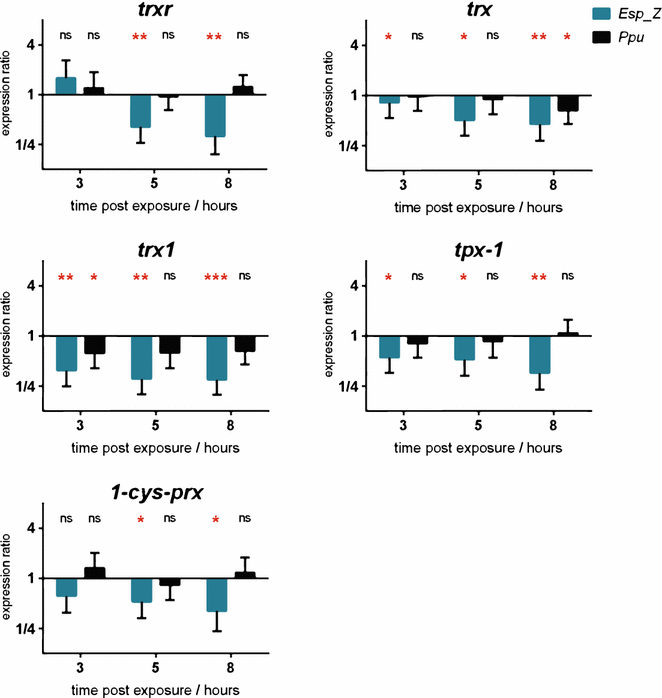
Results: Esp_Z persisted for up to 4 days in the An. gambiae midgut after introduction via nectar feeding, and was able to significantly inhibit Plasmodium sporogonic development. Introduction of this bacterium did not adversely affect mosquito fitness. Candidate genes involved in the selection of a better fit Esp_Z to the mosquito midgut environment and in its ability to condition oxidative status of its surroundings were identified, and parasite expression data indicated that Esp_Z is able to induce a partial and temporary shutdown of the ookinetes antioxidant response.
Conclusions: Esp_Z is capable of inhibiting sporogonic development of Plasmodium in the presence of the mosquito's native microbiota without affecting mosquito fitness. Several candidate bacterial genes are likely mediating midgut colonization and ROS production, and inhibition of Plasmodium development appears to involve a shutdown of the parasite's oxidative defence system. A better understanding of the complex reciprocal tripartite interactions can facilitate the development and optimization of an Esp_Z-based malaria control strategy.
Keywords: Malaria; Microbiota; Mosquito; Oxidative stress; Sugar-bait; Transmission-blocking.
Figures


References
-
- World Health Organization . World Malaria Report 2015. Geneva: World Health Organization; 2016.
-
- Knox TB, Juma EO, Ochomo EO, Jamet HP, Ndungo L, Chege P, et al. An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasit Vectors. 2014;7:14. doi: 10.1186/1756-3305-7-76. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

